
A roundup of company moves and positioning from the pharmaceutical and biotechnology industries and contract service providers.

A roundup of company moves and positioning from the pharmaceutical and biotechnology industries and contract service providers.

The latest on active pharmaceutical ingredients in development and under commercial manufacture

People on the move

The US Food and Drug Administration opened for public comment its Draft Guidance for Industry, ?Cooperative Manufacturing Arrangements for Licensed Biologics.?

Adopting Lean Six Sigma practices in manufacturing and clinical development can help pharmaceutical companies cut costs and bring products to market faster, according to Carmen Medina, principal of Tunnell Consulting.

The US Food and Drug Administration’s Nanotechnology Task Force is urging the agency to develop a guidance and take steps toward addressing nanotechnology-based drugs and medical devices.

Merck & Co. acquires NovaCardia, Sanofi Pasteur completes vaccine-manufacturing facility, more

The US Food and Drug Administration announced the International Conference on Harmonization draft guidance Q10 Pharmaceutical Quality System in the Federal Register (Docket 2007D-0266, CDER 200783).

Beijing Med-Pharm acquires stake in Sunstone, JHP Pharmaceuticals buys King Pharmaceuticals facility, more

Ventana Medical Systems, Inc.'s board of directors unanimously decided that Roche's (Basel, Switzerland) $75 per share cash offer is inadequate in multiple respects and contrary to the best interests of Ventana's stockholders.

The Bosch Group acquires Pharmatech, InterMune enters a supply agreement with Boehringer-Ingelheim, more.

Albany Molecular Research acquires manufacturing sites, Bespak restructures, more
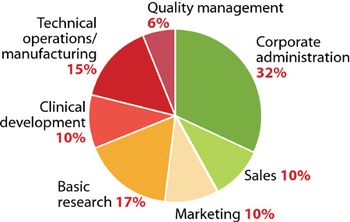
Trenton, NJ (May 18)-Pharmaceutical and medical technology companies in New Jersey have found a striking disparity between six high-demand occupations and the number of qualified workers to fill those positions, according to a report issued by the HealthCare Institute of New Jersey (HINJ). Modest job growth in this field is expected for the next four years, states the report.

Akorn, Catalent, Novo Nordisk, more

Washington, DC (June 22)-Senators Orrin Hatch (R-UT), Edward Kennedy (D-MA), Michael Enzi (R-WY), and Hillary Clinton (D-NY) agreed on legislation that would authorize the US Food and Drug Administration (Rockville, MD) to approve follow-on versions of biologic therapies.

London, UK (June 22)-The competition between biosimilars and branded drugs intensified this week thanks to favorable reviews of three biosimilar products for a popular treatment for anemia.

Althea Technologies partners with GeoVax Labs, Nabi Biopharmaceuticals restructures, more

Rockville, MD (June 18)-The US Food and Drug Administration, the European Commission, and the European Medicines Agency have agreed to extend cooperative activities to the areas of pediatrics and medicinal products for rare diseases (i.e., orphan drugs).

New York (June 19)-The US District Court for the Southern District of New York affirmed the validity and enforceability of Sanofi-Aventis's patent on clopidogrel bisulfate, the active ingredient in the company's coronary artery disease treatment "Plavix."

Cambridge, MA (June 7)-Genzyme Corp. is growing, with a new biomanufacturing plant planned and other expansions nearing completion.

Los Angeles (June 6)-Researchers at the University of California at Los Angeles (UCLA) used silica-based nanoparticles to deliver the anticancer drug camptothecin (CPT) and other water-insoluble drugs to human cancer cells.

Brussels (May 30)-The European Federation of Pharmaceutical Industries and Associations (EFPIA) called for the pan-European and industry-wide adoption of 2-D barcode technology to combat the increase in counterfeit drugs in Europe.


Kvistgard, Denmark (June 4)-Bavarian Nordic received a $1.6-billion contract from the US Department of Health and Human Services to manufacture and deliver 20 million doses of its smallpox vaccine "Imvamune."

AstraZeneca, Barr Pharmaceuticals, Jubilant Organosys, Roche, more

Thousand Oaks, CA (June 4)-Amgen agreed to acquire Ilypsa (Santa Clara, CA), a private company that develops nonabsorbed drugs for renal disorders, for $420 million.

Boston, MA (May 8)-The global biotechnology industry showed several positive signs in 2006, including increases in overall revenues and financing, although the industry as a whole continues to operate at a loss, according to Ernst & Young's (New York, NY, www.ey.com) annual analysis of the biotechnology industry. Its report, "Beyond Borders 2007," was issued at the Biotechnology Industry Organization's (BIO, www.bio.org) annual conference and exhibition, which was held in Boston May 6–9.

Cambridge, MA (May 30)-Genzyme agreed to buy its US partner Bioenvision (New York) in an all-cash transaction worth $345 million.

Rockville, MD (May 25)-The US Food and Drug Administration issued a Form FDA 483, Inspectional Observations, to the Liverpool, United Kingdom, facility of MedImmune, Inc., citing deviations from current good manufacturing practices.
