
Degussa AG (D?sseldorf, Germany) and Lynchem Co., Ltd. (Dalian, Liaoning Province, China) signed a contract to establish a custom manufacturing joint venture.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Degussa AG (D?sseldorf, Germany) and Lynchem Co., Ltd. (Dalian, Liaoning Province, China) signed a contract to establish a custom manufacturing joint venture.

Gilead Sciences, Inc. (Foster City, CA) signed a definitive agreement to acquire the Canadian subsidiary Raylo Chemicals and most of its assets from Degussa AG (D?sseldorf, Germany) for 115.2 million euros ($147 million).

Sandoz (Holzkirchen, Germany), the generics arm of Novartis (Basel, Switzerland), received approval from the US Food and Drug Administration (Rockville, MD) for "Omnitrope" (somatropin [rDNA origin]), a follow-on version of a previously approved recombinant human growth hormone (rhGH) product.

Bristol-Myers Squibb Company (BMS, New York, NY) selected Devens, Massachusetts as the site for its new, large-scale, multiproduct bulk biologics facility.

China?s State Food and Drug Administration (SFDA, Beijing, China) issued a notice on May 18, 2006, requiring drug regulatory departments of provinces, autonomous regions, and municipalities to further strengthen the supervision and management of drug manufacturers.

Schering-Plough Corporation (Kenilworth, NJ) announced plans to phase out manufacturing operations at its Manati, Puerto Rico site, expecting to discontinue operations there by the end of 2006.
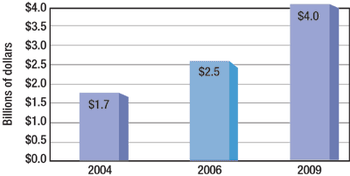
CMOs account for 20–30% of biopharm production. Big Pharma also is filling the biologics supply chain.
The biggest single recent trend in outsourcing solid-dosage processing has been the movement toward discovery and synthesis of more potent active pharmaceutical ingredients.

Xethanol Corporation's (New York, NY) subsidiary CoastalXethanol signed a letter of intent with Pfizer Inc. (New York, NY) to purchase Pfizer's pharmaceutical manufacturing complex in Augusta, Georgia.

Rutgers, the state university of New Jersey (New Brunswick, NJ), the New Jersey Institute of Technology (Newark, NJ), Purdue University (West Lafayette, IN), and the University of Puerto Rico (Mayag?ez, PR) won a $15-million, five-year grant from the National Science Foundation (Arlington, VA) to improve the manufacturing of pharmaceuticals, food, and agricultural products.

Chemir Analytical Services (Maryland Heights, MO) acquired Gateway Chemical Technology, Inc., (St. Louis, MO), a provider of custom synthesis and process development services.

AstraZeneca PLC (London, England) has agreed to acquire the biopharmaceutical company Cambridge Antibody Technology Group PLC (CAT, Cambridge, England).

Merck & Co Inc. (Whitehouse Station, NJ) has agreed to acquire two biologics companies: GlycoFi Inc. (Lebanon, NH), a specialist in yeast glycoengineering for $400 million, and the biopharmaceutical company Abmaxis Inc. (Santa Clara, CA) for $80 million.

Clariant (Muttenz, Switzerland) has agreed to sell its pharmaceutical fine chemicals unit to the private equity firm TowerBrook Capital Partners LP (New York, NY) for CHF 110 million ($89 million). The price includes an earn-out participation of CHF 40 million ($32 million) to be paid in two years.
Indian suppliers of active pharmaceutical ingredients and dosage formulations expand in India, the United States, and Europe.

The biotechnology company Discovery Laboratories Inc. (Warrington, PA, www.discoverylabs.com) reports that analysis of ongoing stability data from "Surfaxin" process validation batches indicates that certain stability parameters have not been achieved, and additional process validation batches will likely have to be produced.

Novartis (Basel, Switzerland) has closed on its $5.4-billion acquisition of Chiron Corporation (Emeryville, CA), paving the way for the creation of a new Novartis division focusing on vaccines and diagnostics.

Sandoz (Holzkirchen, Germany), the generics division of Novartis (Basel, Switzerland) received European marketing authorization for its recombinant human growth hormone, ?Omnitrope? (somatropin), making it the first biogeneric product approved under the biosimilar pathway of the European Commission.

The custom manufacturer Lonza Group (Basel, Switzerland) will invest $200 million in Nansha Guangzhou, China, for production of active pharmaceutical ingredients (APIs) and intermediates.

Total employment in the biosciences in the United States reached 1.2 million in 2004, the latest year for which data are currently available, according to the study, "Growing the Nation's Bioscience Sector: State Bioscience Initiatives 2006," which was released this week at BIO 2006

The global biotechnology industry earned record revenues in 2005 while moving toward profitability as it reduced its collective net losses.

FTC Plans to Study Competitive Impacts of Authorized Generic Drugs

New biocatalytic and chemocatalytic routes to chiral intermediates and developments in simulated-moving-bed and supercritical-fluid chromatography for resolving racemic mixtures.

Global Pharmaceutical Market Shows Moderate Growth

Vertex Pharmaceuticals? CEO Outlines Strategy for Growth

India and China Position for Growth in APIs

GSK Begins RFID Pilot Program

Industry Leaders Call for New Paradigm in Drug Development

BMS, Sanofi-Aventis Settle Over Generic Plavix

Actavis Makes $1.6 Billion Takevoer Bid for Pliva