
To rapidly achieve high-quality pharmaceutical manufacturing processes, industry must develop prospective, management-based approaches instead of retrospective performance-based measures.
Mark Witcher is senior consultant at Brevitas Consulting, witchermf@aol.com

To rapidly achieve high-quality pharmaceutical manufacturing processes, industry must develop prospective, management-based approaches instead of retrospective performance-based measures.

FDA’s 21st Century goals can be realized by using a multi-purpose manufacturing facility with a flexible design that provides reliable production without extensive regulatory oversight.

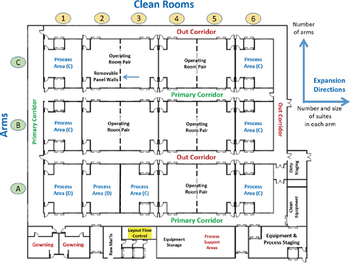
A matrix of multi-functional cleanrooms can be adapted for launching products.

A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

Published: September 2nd 2018 | Updated:
Published: September 2nd 2018 | Updated:

Published: November 2nd 2018 | Updated:

Published: November 29th 2018 | Updated:

Published: July 2nd 2019 | Updated: