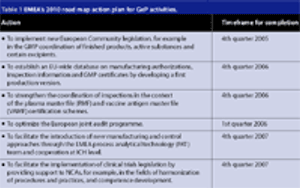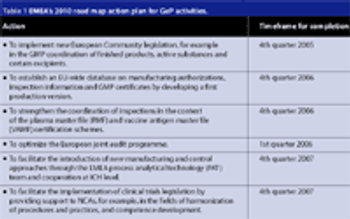
Since the formation of the European Union (EU) in 1993, each member state has brought along its own regulatory baggage, namely the standards and regulations that their companies are formally required to comply with. These standards and regulations still apply for any pharmaceutical products a native manufacturer decides to market within their homeland. When the same manufacturer markets its pharmaceutical products to consumers in other EU member states, the regulatory directives of the European Commission and the European Agency for the Evaluation of Medicinal Products (EMEA) apply as well.