
The approval comes after the results of a clinical trial that showed 61% of patients had a response lasting six months or longer.

The approval comes after the results of a clinical trial that showed 61% of patients had a response lasting six months or longer.

The approval was based on successful data from a years-long trial that assessed patient’s tumor status every 12 or 24 weeks for up to 24 months.

The sNDA was accepted after positive results from a Phase III trial were published in September 2019 that concluded the drug reduced the incidence of cardiovascular death or the worsening of heart failure versus placebo.

The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.

The agency sent a warning letter to Henan Kangdi Medical Devices Co. Ltd after an inspection found CGMP violations that included a variety of failures of the company’s quality unit.

Pressures on FDA will affect industry’s success in bringing new therapies to market.

FDA sent a warning letter to GPT Pharmaceuticals Pvt. Ltd. after inspectors found CGMP violations that included equipment that was not properly maintained.

Aurobindo Pharma USA, Inc. voluntarily recalled Mirtazapine Tablets due to an error on the label that listed the incorrect strength.

FDA’s approval rate slowed, but the US agency is still ahead of its international counterparts in green-lighting new drugs for market.

In 2020, European regulators are expected to start to be even more active in encouraging drugs innovations rather than hindering them through legal restrictions.

As a new decade has begun, this editorial reviews some of the biggest, brightest, and boldest happenings from the industry over the past 10 years.
Regulatory mandates, niche diseases, and patient-centric solutions have all impacted pharma packaging over the years and are expected to help shape the future of the sector.

Without careful consideration and understanding, new regulations for medical devices could lead to the withdrawal of combination products from the market.

The guidance describes procedures for obtaining an additional National Drug Code for prescription drugs imported into the United States.

Glenmark Pharmaceuticals is recalling unexpired lots of Ranitidine Tablets due to potential presence of N-nitrosodimethylamine (NDMA).

FDA sent a warning letter to Dercher Enterprises, Inc., DBA Gordon Laboratories, for CGMP violations and adulterated drug products.

The European Medicines Agency and its European partners have launched a pilot program for cooperation in the inspection of facilities that manufacture sterile drug products.

The revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world are described.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An understanding of global and national pharmacopoeias is crucial to understanding change processes and access to different markets.
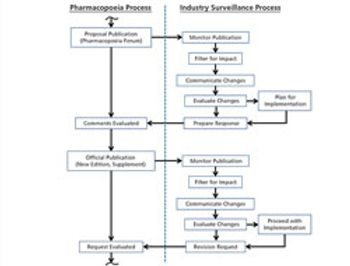
The process used to monitor and participate in pharmacopoeial changes is described.

Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.

Warning letters tell the tale of missteps by drug companies and offer a path to compliance for quality teams that monitor these enforcement actions.