
The US Food and Drug Administration last week posted the final version of its Guidance for Industry, ANDAs: Impurities in Drug Substances.

The US Food and Drug Administration last week posted the final version of its Guidance for Industry, ANDAs: Impurities in Drug Substances.

Two years ago, the US Food and Drug Administration's Office of Generic Drugs (OGD) developed and implemented a program to test question-based reviews for abbreviated new drug applications (ANDAs). OGD is reviewing the benefits and challenges faced by pharmaceutical firms involved in the initiative to see what changes need to be made going forward.

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

The US Food and Drug Administration issued a draft guidance for industry, Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting, on July 13.

The pharmaceutical industry is paying more attention to less well-known tropical diseases, according to a report from the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA).

The Society of Chemical Manufacturers and Affiliates (SOCMA) this week stated its support to the US Senate for approving legislation that would extend existing chemical security standards for one more year.

This week, the Biotechnology Industry Organization (BIO) and the Generic Pharmaceutical Association (GPhA) issued their opposing statements in reaction the July 13 passage of an amendment from the US Senate Committee on Health, Education, Labor, and Pensions (HELP) regarding the creation of a pathway for the approval of new competitors of biologic drugs.

The US Pharmacopeia is revising its monographs for four pharmaceutical excipients: propylene glycol, sorbitol solution, sorbitol sorbitan solution, and noncrystalllizing sorbitol solution.

Roche has officially withdrawn from the Pharmaceutical Research and Manufacturers Association (PhRMA) after being a member for 36 years.

The Federal Coordinating Council for Comparative Effectiveness Research recommended that data infrastructure be the primary investment for the US Department of Health and Human Services's (HHS) comparative-effectiveness research (CER) funds.

On June 25, US Marshalls seized all drug products and ingredients at three facilities of Caraco Pharmaceutical Laboratories.

The UK's Medicines and Healthcare Regulatory Agency (MHRA) has published the outcome of a consultation on measures to strengthen the country's drug supply chain.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

Some GMP agents seem to find a way to squander time, money, and common sense.

Is it good policy to pay for bad behavior?

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.
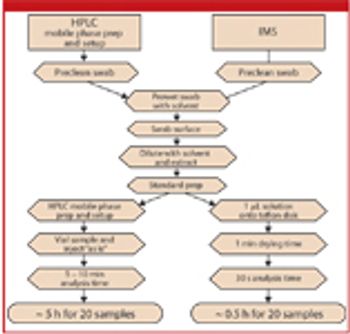
The authors discuss the theory of ion mobility spectrometry, its benefit over HPLC analysis in cleaning verification, and the experimental considerations for method validation and validation.

The Physician Payments Sunshine Act is still pending before the US Senate Committee on Finance, according to Jill Kozeny, communications director for Senator Chuck Grassley (R-IA).

Former Senator Majority Leaders Howard Baker, Tom Daschle, and Bob Dole released a report this month, Crossing Our Line: Working Together to Reform the US Health System, which proposes four pillars of health reform.

Following the 2008 launch of the European Fine Chemicals Group (EFCG) Voluntary Guidelines (VGs) to promote supply-chain security, the group has now announced the official launch of an assessment template that will allow fine chemicals customers and suppliers to assess and implement this new set of recommendations.

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

Last week, the steering committee and expert working groups of the International Conference on Harmonization met in Yokohama, Japan, to discuss pending guidelines and other relevant issues.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.