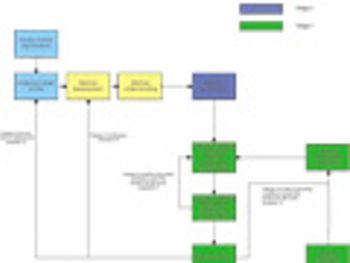
The authors describe how traditional approaches to analytical method and validation may benefit from alignment with quality-by-design concepts.

The authors describe how traditional approaches to analytical method and validation may benefit from alignment with quality-by-design concepts.

Only the strong survive when it comes to pharmaceutical packaging and shipping.
This study examines the effect and interaction of variations in hypromellose physicochemical properties.

Ties between the biotechnology industry and university research are crucial.

International trade can be great for business, but breaking border laws can put one in hot water.

Meticulous system configuration can prevent machines from taking over.

The author discusses how a CDMO helps in gaining process understanding and in developing robust, high-quality products and processes.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.

Careful attention to detail will help to prevent valuable assets from "melting" away.

An understanding of the pan-coating process based on first principles can support successful scale up.

Even the slightest of errors in exponential calculations can cause the biggest of headaches.

A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.

It's better to catch costly mistakes in the laboratory before they reach the accounting department.

Industry experts working with extended-release injectables discuss challenges and solutions to formulating and manufacturing these complex products.

There are various theories about how to scale up a solid dosage coating operation in a pan coater. This article provides a basic process understanding and scale-up theory based on first principles.

Critical issues that should be considered when scaling up a hot-melt extrusion process.

Failure to disclose info may work sometimes, but eventually every question will be answered.

In a world where product recalls can mean the end of a company, all batches must be perfect.

With the increasing use of hot-melt extrusion, extruder manufacturers have developed mid-size twin-screw extruders that facilitate process development and scale-up to commercial processes.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

Technology may expedite operations, but the absence of the human element could cost dearly.

Cleanliness is crucial, even if zapping and trapping is necessary to reduce product contamination.

On Sept. 27, 2011, FDA sent Genentech a Form 483 listing several violations at the company's South San Francisco, California, plant. The violations included problems with investigations into batch failures, inappropriate equipment design, and insufficient protection against contamination. FDA visited the plant, which produces the cancer drug Avastin, 13 times in September 2011 and made four observations.

Careful mixing during a product's distillation can help avert trouble from a strong concoction.

June and July saw three major US conferences on implementing single-use technologies: the IBC Single-use Applications meeting, the PDA Single-use Workshop and the Bio-Process Systems Alliance (BPSA) International Single-use Summit (ISUS). Jerold Martin highlights some of the key topics discussed at these meetings.