
Watson-Marlow and Franz Ziel GmbH have launched a new integrated solution to fast-track cell, gene, and biological therapies.

Watson-Marlow and Franz Ziel GmbH have launched a new integrated solution to fast-track cell, gene, and biological therapies.

Grand River Aseptic Manufacturing has completed phase II of its facility expansion with the installation of two new sterile filling lines.

Point-of-use manufacturing may lead to a big change in the accessibility of medicines globally.

Getinge’s new DPTE-EXO with Sleeveless DPTE-BetaBag is an alpha port with an external opening and an integrated funnel for automated aseptic transfer.

MilliporeSigma’s new BioContinuum Seed Train Platform offering enables a fully closed bioprocessing environment for both fed-batch and perfusion N-production.

Merck has entered into a collaboration with Agilent Technologies with the aim of filling the industry gap in PAT for downstream processing.

This article explores the concerns with cleaning pharmaceutical products utilizing LNP delivery vehicles and provides a general cleaning recommendation based on laboratory and field testing.
Flexible and efficient methods are needed for biopharmaceutical manufacturing.

By following some fundamental steps, manufacturers can optimize their tablet tooling maintenance and resulting productivity.

The struggle to implement continuous biomanufacturing at GMP level is slowly advancing.

The new company has been appointed as a channel partner for Ireland by TSI Inc., a particle counter manufacturer.

Digital maintenance solutions can help visualize the value and key activities provided by the equipment vendor from inception to utilization.

Exhibitors display primary packaging, secondary packaging, and machine innovations at this year’s INTERPHEX.

INCOG Biopharma has completed the construction of its cleanroom production area, marking a milestone in the completion of an overall $100 million investment in a new facility.

Collaboration and new tools aid efforts to implement new processing technologies for small-molecule drug product manufacturing.

The continuous, aseptic fill/finish process is finding use in vaccines and biologic drugs.

Thermo Fisher Scientific’s new Gibco CTS Xenon Electroporation System aims to provide easier scale up for cell therapies, from clinical development to commercial manufacturing.

Curia and the US government have entered into a cooperative agreement to expand fill/finish capability for injectable medicines.

Smart manufacturing transforms management of tablet and capsule equipment and processes.

The ROSS X-Series Inline Ultra-High Shear Mixer produces quality dispersions, suspensions, and emulsions in a variety of industries.
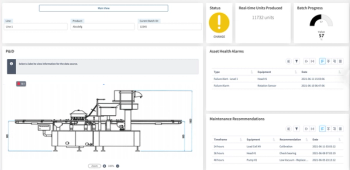
Aizon's Asset Health Application system is customized to manage each manufacturer's unique asset maintenance needs.

Linkam Scientific’s RHGen Relative Humidity (RH) controller allows for precise control of sample humidity without need for an external dry air supply.
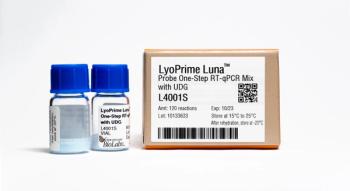
New England Biolabs Luna Probe One-Step RT-qPCR Mix with UDG enables sensitive, linear, real-time detection of target RNA sequences.

Moderna and Thermo Fisher Scientific have formed a collaboration to leverage dedicated commercial fill/finish manufacturing capacity in the US for mRNA vaccines and therapies.

Hoffman Neopac has signed a partnership with Saperatec to begin recycling aluminum laminate composites upon its opening in mid-2023.