Formulation and Drug Delivery
Latest News
Latest Videos

More News

A coordinated and international response is needed to help control the latest mpox outbreak in Africa.

Employing novel technologies and more patient-centric approaches can help to reduce the potential of formulation failure.

Biopharmaceutical production faces the challenge of ensuring the quality of raw materials due to a lack of specific guidelines. By implementing effective risk assessment strategies and working with reliable, selected solution providers, biopharmaceutical manufacturers can minimize these challenges and improve product quality.

A holistic approach to automation can provide benefits at all stages of development and manufacturing.

STADA and Alvotech have launched Uzpruvo in Europe, making it the first approved biosimilar to Stelara (ustekinumab) in the European market.

Pelletization, a popular oral drug delivery method in pharmaceuticals and nutraceuticals, offers numerous benefits. This article delves into the critical parameters for formulation development and technological consideration in pelletization, elucidating its significance in advancing pharmaceutical solutions.
Weighing development costs/resources and performance benefits is essential.

Roche voluntarily recalled Susvimo’s ocular implant, insertion tool, and initial fill kit when test results did not satisfy company standards.

The companies have restructured their existing collaboration into a licensing agreement that invests in mRNA development.

A greater number of patients with Duchenne muscular dystrophy will be able to be treated after FDA's approval of a gene therapy.

The company is referring to its search for innovative approaches as crowdsourcing and will award at least one cash prize.

At BIO 2024 in San Diego, John Dunlop, PhD, chief scientific officer at Aliada Therapeutics, sat down for an interview with Pharmaceutical Technology to discuss the firm’s MODEL platform and its potential impact on therapeutic delivery for brain-related conditions.

CordenPharma has partnered with Spain-based Certest to develop ionizable lipids for LNP formulations.
A statistical analysis for determining an expiration date can be applied to replicates or their corresponding averages as suggested in industry guidelines.

Artificial intelligence and machine learning can help overcome poor solubility and bioavailability.

The final drug product relies on the quality and reliability of the raw materials used in its production.

Biosimilar analytical assessments focus on demonstrating biosimilarity and interchangeability with the reference biologic.
A combination of rapid sterility methods with the industry standard compendial method should ensure maximum safety.

Bkemv (eculizumab-aeeb) is the first interchangeable biosimilar to Soliris (eculizumab) to treat paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.

In an ASGCT Panel on nucleic acid- and cell-based vaccines in oncology, work on a personalized mRNA vaccine was highlighted.

The partnership aims to provide end-to-end development and manufacturing for biopharmaceutical drug substance and drug product.

At CPHI NA, Rachel Harris, AstraZeneca, spoke on her work at BioPhorum to enable industry consensus and action for sustainability with drug delivery devices.

The company’s presentation at ASGCT includes preliminary data results for a child who received the gene therapy.

A greater understanding of molecular and cellular biology and technological advancements are revolutionizing the field of biologics.

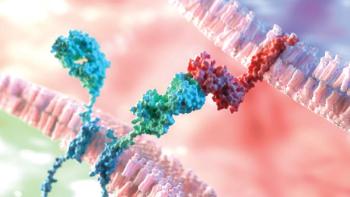
Novel delivery technologies enhance brain penetration to target neurodegenerative diseases and glioblastomas.