Manufacturing
Latest News
Latest Videos

More News

Large-language models are excellent for general-use AI systems, but they don’t understand pharmaceutical companies’ proprietary documentation—the validated procedures and quality protocols that ensure drug safety. Smaller, domain-specific language models give companies more control and efficiency in their AI use.

Starting with risk mitigation, GMP facility and equipment maintenance is all about anticipating problems before they happen and showing regulators a controlled environment.

3D printing enables personalized precise drug delivery, enhances compounding efficiency, and is driving new regulatory models like distributed pharmaceutical manufacturing.

Webinar Date/Time: Tue, Dec 9, 2025 11:00 AM EST

Take our survey to voice your opinions on the bio/pharma industry impacts of the FDA Commissioner’s National Priority Vouchers program.

Webinar Date/Time: Thu, Nov 20, 2025 12:30 PM EST

Webinar Date/Time: Fri, Nov 21, 2025 11:00 AM EST

Thermo Fisher’s Jennifer Cannon reviews the first year of the company’s Accelerator Drug Development suite of services and the overall experience of attending the CPHI conference.

Pharma companies are focusing on sustainability efforts to meet client expectations, environmental audits, and climate pressures.

The planned Kenvue acquisition, expected to close in the second half of 2026, has notable implications for pharmaceutical R&D and manufacturing professionals.

Sriman Banerjee of Takeda Pharmaceuticals says patient adherence is improving thanks to technologies that offer a more personalized approach.

SGD Pharma’s Najet Mebarki provides an overview of more than a half dozen services, products, and treatments, including ready-to-use solutions.

SK pharmteco boosts US peptide synthesis and purification capabilities, advancing reliable large-scale manufacturing for biopharma innovation.

Webinar Date/Time: Tue, Nov 18, 2025 11:00 AM EST
At CPHI and AAPS, Ashland plans to detail the impacts of its advanced excipients: ultra-low nitrite materials curb nitrosamine risk, while high-purity sucrose stabilizes complex biologics.

Take this quiz to test your comprehension of one of our recent feature articles.


The company will also highlight its sustainability strategies and quality solutions at the event.

Webinar Date/Time: Tue, Nov 4, 2025 12:00 PM EST

This issue showcases strategies that organizations are adopting to stay competitive as pressing industry trends shape the sector.

AstraZeneca’s $4.5 billion investment in Virginia expands its US drug manufacturing footprint, creating 3600 jobs and integrating AI for cancer and metabolic therapies.

The increasing diversity and complexity of injectable drug products is driving innovation.

Webinar Date/Time: Wed, Nov 5, 2025 11:00 AM EST

Webinar Date/Time: Wed, Nov 12, 2025 11:00 AM EST
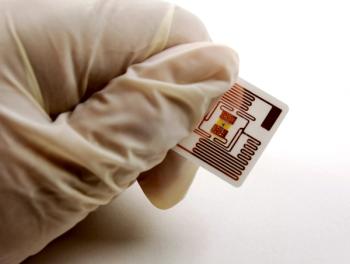
The new label for prefilled syringes, in conjunction with innovations from SCHOTT Pharma, enables integrated tamper-evidence and digital tracking across the supply chain.