Manufacturing
Latest News
Latest Videos

More News

BioPharm International® sat down with Noah Kopcho, field application scientist at Gyros Protein Technologies, to talk about the challenges in the production of AAV vectors.

Incorporating sustainable practices into process designs as early as possible ensures optimal performance.

The shift toward personalized medicines poses new challenges in cleanroom protocols.

Webinar Date/Time: Thu, May 22, 2025 11:00 AM EDT
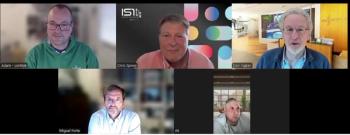
Donald Ingber, MD, PhD; Miguel Forte, MD, PhD; Ali Pashazadeh, MRCS, MBA; and Adam Inche, PhD, MBA go behind the headlines to examine the fast-changing landscape of manufacturing re-shoring, tariffs, NIH budget cuts, and the outlook for cell and gene therapies.

The company’s exhibitions will be focused in the areas of its pipeline, proprietary capsid engineering, and manufacturing.


The commitment will include new, state-of-the-art R&D facilities as well as new or expanded manufacturing sites in multiple states.


Calculating the amount of drug needed for compounding requires different considerations for respective drug types and forms.

On April 14, 2025, the Trump Administration launched a national security-driven investigation into pharmaceuticals, a move that will likely result in tariffs being placed on pharmaceutical drugs, ingredients, and other components that are imported from outside of the United States.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about the use of artificial intelligence and machine learning in pharmaceutical development and manufacturing.

Development of the state-of-the-art facility was made possible by a £2 million (US$2.6 million) round of funding, and a successful inspection by MHRA was completed in March 2025.

The company has plans to invest $23 billion during the next five years to expand manufacturing and R&D in the US, which will include seven new facilities.

Pharmaceutical Technology® Group spoke with Erik Wiklund, CEO of Circio, about the impact of the post-COVID-19 world on the pharmaceutical industry and how that has shifted the talent pool.

Siemens said spending on AI software is predicted to double by the end of the 2020s, in a market increasingly driven by structural shifts that underscore a need for digital transformation.

SkyCell is working with Microsoft to incorporate SkyCell’s AI-powered supply chain solution into Microsoft Teams and Copilot.

Lack of skills in the AI realm was a distant second among those surveyed about the biggest barrier to innovation while using the technology.

Joe Bobacher of OPW Engineered Systems spoke with Pharmaceutical Technology® Group at INTERPHEX 2025 about fluid transfer in controlled environments and the importance of guarding against product loss.

As single use becomes more prevalent, end users are looking for more customization, and regulators are refining their rules, says Todd Andrews of CPC in an interview with Pharmaceutical Technology® Group.

3D printing of personalized medications is currently possible under existing compounding regulations, offering enhanced process control through automation. But new legislation coming in 2025 will allow 3D printing as part of a distributed manufacturing framework.

Michael Franco, global sales director at PSG Biotech, spoke with the PharmTech Group at INTERPHEX 2025 about recent advancements in pharmaceutical flow sensor technology.

The yearly convention in New York City showcased new and emerging technologies and gave participating exhibitors a chance to unveil their strategies for the second half of the 2020s.

Josh Hoerner, general manager of Purisys, provides his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.

The move towards “pharma 4.0” requires a major shift, both ideologically and technologically, to adapt current processes to a framework that will automate much of today’s manufacturing.