
In a three-part series, the author explores the potential use of agentic AI in pharmaceutical R&D.

In a three-part series, the author explores the potential use of agentic AI in pharmaceutical R&D.

Advancements for novel cancer therapies are booming. This video takes a look at some of the latest efforts in the cancer research and therapy arena.

EMA states the new variations guidelines will streamline lifecycle management and make processing variations quicker.

While plastics have revolutionized pharmaceutical packaging, that evolution within the industry has come at a cost.

In the first-ever “Two-Minute Mysteries: BioPharma Stories,” Agilent’s Ken Boda shares the mystery of a low reading.

The week's pharma news includes FDA warnings for Lilly and Novo, new drug approvals for MASH and edema, and a look ahead at CPHI Europe.
CDC panel shifts vaccine policy by discouraging combined MMRV shot for young children due to increased febrile seizure risk.
In this exclusive Drug Digest video, Steve Barr from SK pharmteco and Prasad Raje from LGM Pharma explore how supply chain pressures, sustainability, and AI adoption are reshaping small molecule development and excipient use in pharma.

Finalists for CPHI Frankfurt Pharma Awards 2025 showcase innovation in drug development, advanced manufacturing, and future industry leaders.

The company, under parent ICE Pharma, will celebrate its past achievements, present its expertise and plans for the future, and unveil a “100 Years” logo.

Pharmaceutical Technology® spoke with Todd Sprouse, associate director, Analytical Services, and Erik Feldmann, PhD, principal technical advisor, Client Solutions & Proposals, both with Cambrex, to find out more about the challenging situation.

Dan Williams, CEO of SynaptixBio emphasizes how small biotechs are using genetic research and partnerships to accelerate rare disease innovation in the final installment of his interview.
The Phase IIa study will test HTL0039732 in combination with immunotherapy to boost responses in resistant solid tumors and broaden treatment choices.

The company has expanded its CDMO offerings in recent years, adding to decades of experiences in solutions such as moisture management.
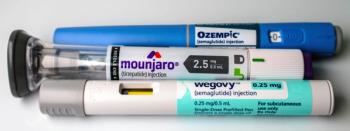
Misleading promotions of GLP-1 and compounded semaglutide products are drawing renewed regulatory scrutiny over risk disclosure and safety messaging.
Corstasis Therapeutics plans to launch the product in the United States in the fourth quarter of 2025.

Novo Nordisk’s Wegovy will be competing with Madrigal Pharmaceuticals’ Rezdiffra in the metabolic dysfunction-associated steatohepatitis arena.

UK pharma small- and medium-sized enterprises face funding and R&D barriers. Improved tax relief and targeted incentives are vital to keep UK innovation competitive.

The 2024 edition of the annual conference, held in Milan, attracted more than 59,000 professionals from across the bio/pharmaceutical landscape.

This potential shift has profound implications for regulatory strategy, transparency, and internal quality systems, directly impacting the core concerns of industry professionals.

Pharma embraces AI for quality, supply chain, and training; navigates FDA compliance; and develops new treatments like an epinephrine nasal spray.

The two organizations are collaborating on an integrated translational biology platform for the development of radiopharmaceuticals.

Bram Baert, global head of Regulatory Affairs at Lonza CHI, gives his perspective on the impact of the EC’s decision on the use of TiO2 in drug products.

Merck’s Sanat Chattopadhyay called for stronger leadership, data-driven oversight, and shared accountability to raise pharmaceutical quality standards.

Susan J. Schniepp, Distinguished Fellow, Regulatory Compliance Associates, joins PDA CEO Glenn Wright and Melissa Seymour, Executive Vice President, Global Quality, Eli Lilly and Company, to talk about the regulatory and quality topics discussed at the 2025 PDA Regulatory Conference.

AI can offer a strategic blueprint for GxP compliance, risk mitigation, and human-led operational excellence.

FDA experts at PDA 2025 urges pharma to adopt holistic quality systems, strengthening 483 responses & post-warning letter remediation for compliance excellence.

Charles Gibbons of Lachman Consultants and Michael Grischeau of AbbVie stressed AI governance, data integrity, and human oversight as essential to applying digital tools across labs and supply chains.

Scientific discovery, technological evolution, and market demands are constantly reshaping the landscape.

Aaron Cowley, Recipharm Advanced Bio; Renee Hart, LumaCyte; and Vibha Jawa, EpiVax, go behind the headlines to delve deeper into recent market deals driven by Big Pharma patent cliffs and the complex manufacturing of ATMPs.