
The Pharmaceutical Research and Manufacturers of America and Biotechnology Industry Organization released statements last week regarding the Senate's vote to pass the Food and Drug Administration Safety and Innovation Act, which reauthorizes PDUFA.

The Pharmaceutical Research and Manufacturers of America and Biotechnology Industry Organization released statements last week regarding the Senate's vote to pass the Food and Drug Administration Safety and Innovation Act, which reauthorizes PDUFA.

A recent report from the UK's All Party Pharmacy Group has attributed shortages of prescription medicines in the UK to parallel trade and is calling for urgent action.

GSK Agrees to Acquire Cellzome; Patheon Restructures; and More.

The US Pharmacopeia has issued a third round of improvements to its blood thinner standard.

PDA Revises Technical Report on Sterilized Products

The Parenteral Drug Association has released guidance on the detection and mitigation of 2,4,6-tribromoanisole and 2,4,6-trichloroanisole taints and odors in pharmaceutical and healthcare products.

Human Genome Sciences rejects GlaxoSmithKline offer to acquire the company and issues a poison pill to prevent a takeover.

AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen Pharmaceutica NV, Merck Serono, and Pfizer have pledged support of more than £14 million ($23 million) to a translational drug development project being run by the UK's Medical Research Council and the University of Dundee.

GSK Agrees to Acquire Cellzome; Patheon Restructures; and More.

We are using a vacuum oven to dry our wet-cake intermediate. At 120 °C, it takes 26 hours on average to dry the material from 80% to 10% moisture. Can we shorten our drying time, possibly by agitating the product to improve heat transfer?

Senators Max Baucus (D-MT) and Chuck Grassley (R-IA) send letters to opioid manufacturers and pain groups asking them to disclose financial ties.

ICH Q11, the anticipated guideline from the International Conference on Harmonization, titled Development and Manufacture of Drug Substances, has achieved international consensus. Q11 has been one of the fastest guidelines to move through the ICH harmonization process.

The Center for Drug Evaluation and Research Ombudsman's office released its annual report detailing the common questions and complaints received by the office in 2011. A total of 461 inquiries was received, an increase of 11% from 2010. The majority of inquiries, 60%, originated from industry, with consumers accounting for 23%, healthcare providers 7%, FDA employees 5%, and other 5%.

GlaxoSmithKline tenders offer of $13.00 per share in an unsolicited bid despite an earlier rejection by Human Genome Sciences.

Coherus BioSciences, Daiichi Sankyo Form Biosimilar Pact; Sandoz Agrees to Acquire Fougera Pharmaceuticals; and More.

The European Federation of Pharmaceutical Industries and Associations has formally adopted a memorandum of understanding with key partners for a harmonized, European system for medicines verification.

Abbott Laboratories will pay $1.6 billion to settle outstanding issues concerning past sales and marketing practices for its neurologic medication, Depakote.

FDA issued a final rule on sterility testing on May 3, 2012, which amends the requirements for most licensed biological products and aims to provide manufacturers with the flexibility, as appropriate, to keep pace with technological and scientific advances. Many steps are changed or eliminated.

Sanofi has received FDA and EMA approval for expansion of fill and finish operations for alglucosidase alfa.

The UK's Medicines and Healthcare products Regulatory Agency has launched a new anticounterfeiting strategy with the aim of curbing the occurrence of falsified medicines in the county's supply chain.

AstraZeneca's CEO David Brennan To Retire; PRA, Amgen Reach New Biosimilar Agreement; and More.

Clinical research organizations see reform in clinical-trial process, including the establishment of chief innovation officer at FDA.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations

It's better to catch costly mistakes in the laboratory before they reach the accounting department.

Industry leaders share their perspectives on how the pharmaceutical industry is and can further advance sustainability.

The company provides progress made in 2011 and future environmental objectives.

The Obama administration details five strategic imperatives to drive bioscience research as a means of economic growth.
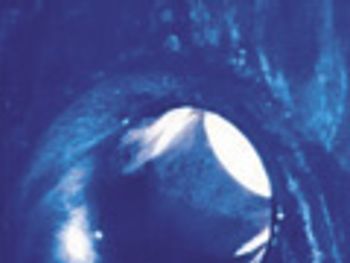
Equipment design and cleaning procedures both play a role in thorough sterilization and cleaning.

At the request of FDA, the IOM prepared a report containing recommendations for how FDA might better monitor the safety of drugs after they have been approved.

Apple's experience with manufacturing facilities in China present opportunity for future best practice.