
Alan Sheppard, Principal, Global Generics at IMS Health and CPhI expert panel member, discusses generic medicine opportunities in the 2016 CPhI Annual Industry Report.

Alan Sheppard, Principal, Global Generics at IMS Health and CPhI expert panel member, discusses generic medicine opportunities in the 2016 CPhI Annual Industry Report.

Three-dimensional (3D) printing, which is a type of additive manufacturing (AM), enables fabrication of specialty drugs and medical devices, said Emil Ciurczak, Doramaxx Consulting and CPhI expert panel member, in the 2016 CPhI Annual Industry Report.

The CDMO has acquired a new building to expand its high-containment manufacturing and development operations.

Sharp will present alongside Tracelink, Systech, Covectra, and Domino during CPhI Worldwide.

Mettler Toledo launched the Rainin BenchSmart 96, a new liquid handling platform.

STERIS launched the Reliance 300XLS Laboratory Glassware Washer, adding to the company’s line of glassware and plasticware washing systems.

Onset expanded its product line for pharmaceutical cold-chain management with the launch two new products.

The most recent PharmSource report says bio/pharma companies have spent more than $150 billion for new plant equipment in the past five years.

BASF’s new production facility in Shanghai will produce PVP K30 powder, a polymer used as a base for pharma excipients.

The agency approves Amjevita (adalimumab-atto) for the treatment of inflammatory diseases.

FDA and EMA set up new working group on the development of treatments for rare diseases.

Sharp Packaging Services adds Biotechnology Center of Excellence to its to its Allentown, PA campus.

Pfizer announced its decision to remain one company after debating the possibility of splitting Pfizer Innovative Health and Pfizer Essential Health into two, separate publicly traded companies.

HHS entered into a $43.18 million contract with Sanofi Pasteur for the development of a Zika vaccine candidate.

Wells Pharmacy Network is voluntarily recalling all of its products due to sterility concerns.

Hebei Yuxing Bio-Engineering Co. Ltd. was cited for data integrity violations.

The company will add a new clinical services facility at Central Park on Bridgend Industrial Estate.

NICE estimates asfotase alfa will cost £367,000 per patient per year.

The agency published the guidance to help generic-drug facilities comply with the GDUFA self-identification requirement.

R-Pharm facility in Yaroslavl, Russia, is designed to produce biological drugs with GE Healthcare's FlexFactory manufacturing platform.

Dow and Colorcon extend and broaden the scope of the Controlled Release Alliance.

Researchers from the Wyss Institute explain a potential method for transporting and producing temperature-sensitive pharmaceuticals at a reduced cost.
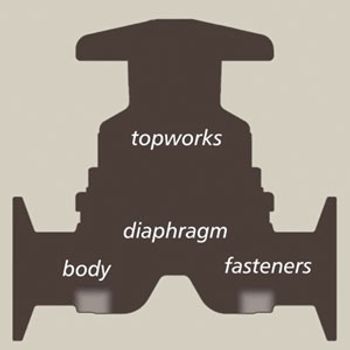
A process-specific preventative maintenance program improves productivity and reliability.

The company announced that an August 2016 FDA inspection of the company’s facility resulted in no form 483s.

The agency has confirmed that patients who take plasma- or urine-derived drugs are not at increased risk for Zika.

Genentech’s biologics drug substance plant is the overall winner of the International Society for Pharmaceutical Engineering’s 2016 FOYA Awards.

Clinical biotechnology company Moderna Therapeutics will build an integrated clinical manufacturing facility for mRNA production in Norwood, Massachusetts.

CRT and SV Life Sciences launched Artios Pharma, a new company formed to develop drugs targeting the DNA damage response to cancer.

Mucodel reported that the study met its objectives, and effectively delivered a dose of naloxone using an oromucosal route.

The companies will collaborate on mRNA-based cancer vaccine development.