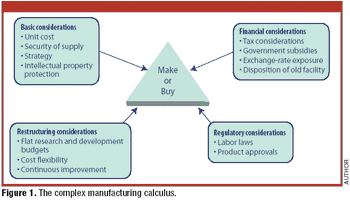
PTSM: Pharmaceutical Technology Sourcing and Management
Candid comments from a Big Pharma executive highlight the complexity of contract manufacturing.

PTSM: Pharmaceutical Technology Sourcing and Management
Candid comments from a Big Pharma executive highlight the complexity of contract manufacturing.
PTSM: Pharmaceutical Technology Sourcing and Management
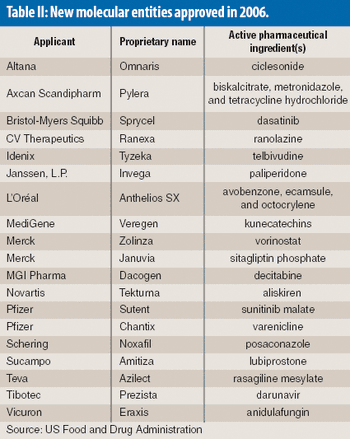
The number of new molecular entities approved by the US Food and Drug Administration is down this year as the pharmaeutical industry continues to face an innovation drought.

PTSM: Pharmaceutical Technology Sourcing and Management
Big Pharma's strategic push into biologics and continuing restructuring are among the highlights for 2007.

PTSM: Pharmaceutical Technology Sourcing and Management
Chi-wan Chen, deputy director of the Office of New Drug Quality Assessment (ONDQA) at the US Food and Drug Administration's Center for Drug Evaluation and Research, shares insight from FDA's pilot program that was designed to allow pharmaceutical companies to submit CMC information demonstrating application of quality by design.