
PTSM: Pharmaceutical Technology Sourcing and Management
A look at PharmaCheck, a field-based tool in development that uses microfluidic chip technology for assessing the quality of medicine in the developing world.

PTSM: Pharmaceutical Technology Sourcing and Management
A look at PharmaCheck, a field-based tool in development that uses microfluidic chip technology for assessing the quality of medicine in the developing world.

PTSM: Pharmaceutical Technology Sourcing and Management
Although serving niche areas, orphan drugs offer good market opportunities for pharmaceutical companies and their suppliers.
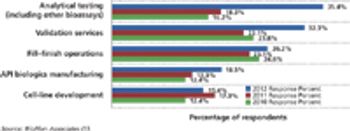
PTSM: Pharmaceutical Technology Sourcing and Management
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

PTSM: Pharmaceutical Technology Sourcing and Management
The Generic Drug User Fee Act seeks to improve and enhance regulatory activities, including achieving parity of inspections between foreign and domestic drug-manufacturing sites for both finished dosage forms and APIs of generic drugs.

PTSM: Pharmaceutical Technology Sourcing and Management
A roundup of the latest developments for drug delivery of parenteral drugs.

PTSM: Pharmaceutical Technology Sourcing and Management
A roundup of developments in corporate social responsibility (CSR) and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.