
PTSM: Pharmaceutical Technology Sourcing and Management
Innovative new technologies released over the past several months seek to enhance bio/pharmaceutical development and manufacturing.

PTSM: Pharmaceutical Technology Sourcing and Management
Innovative new technologies released over the past several months seek to enhance bio/pharmaceutical development and manufacturing.

PTSM: Pharmaceutical Technology Sourcing and Management
Advances in equipment, instrument, and control systems are enabling online monitoring of continuous API manufacturing.
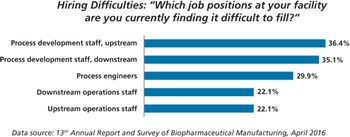
PTSM: Pharmaceutical Technology Sourcing and Management
Contract manufacturing organizations may fill manufacturing gaps created by a lack of trained workers at Chinese biopharma companies.

PTSM: Pharmaceutical Technology Sourcing and Management
New incubators, instrumentation, and staff will increase cell bioassay capabilities for EAG Laboratories.

PTSM: Pharmaceutical Technology Sourcing and Management
The company increased capabilities at its Pharmatek San Diego facility in response to market demand for solubility enhancement solutions.

PTSM: Pharmaceutical Technology Sourcing and Management
The new suite will be used to produce non-sterile dosage forms, such as metered dose inhalers and semisolid topical products, for clinical studies up to Phase II.

PTSM: Pharmaceutical Technology Sourcing and Management
The company said it plans to invest $130 million in the United States and the United Kingdom to increase production capacity.

PTSM: Pharmaceutical Technology Sourcing and Management
Pharmaceutical Technology spoke with Brad Pedrow and Rajesh Singh of Deloitte Consulting to discuss serialization implementation, and what to expect as the DSCSA deadline approaches.

PTSM: Pharmaceutical Technology Sourcing and Management
Cobra will increase capacity in response to customer demand for DNA and viral vector production.

PTSM: Pharmaceutical Technology Sourcing and Management
The company announced the expansion of its global shipping program, which now allows companies to ship dangerous goods between 36 countries.

PTSM: Pharmaceutical Technology Sourcing and Management
Sharp acquired the pharmaceutical packaging facility in Bethlehem, PA.

PTSM: Pharmaceutical Technology Sourcing and Management
Uhlmann Packaging Systems joined the OPC Foundation’s Open Serialization Communication Standard (OPEN-SCS) Working Group.

PTSM: Pharmaceutical Technology Sourcing and Management
Fisher BioServices will expand its CryoHub solution by co-locating it with the Cell and Gene Therapy Catapult manufacturing center for seamless supply chain management and to accelerate cell and gene therapy production.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA sent a warning letter to Lumis Global Pharmaceuticals Co. Ltd. detailing CGMP deficiencies regarding API repackaging, labeling, and misbranding.

PTSM: Pharmaceutical Technology Sourcing and Management
Transparency between pharmaceutical companies and suppliers and risk assessment efforts are vital to effective supply chain practices.