
PTSM: Pharmaceutical Technology Sourcing and Management
Xcellience receives approval to handle both analytical and manufacturing of DEA Scheduled products.

PTSM: Pharmaceutical Technology Sourcing and Management
Xcellience receives approval to handle both analytical and manufacturing of DEA Scheduled products.

PTSM: Pharmaceutical Technology Sourcing and Management
A consensus-based standard issued by NSF International incorporates regulatory and industry requirements into a single standard for the manufacturing and distribution of pharmaceutical excipients.

PTSM: Pharmaceutical Technology Sourcing and Management
As an affiliate member, TraceLink brings serialization expertise to the Pharma & Biopharma Outsourcing Association.

PTSM: Pharmaceutical Technology Sourcing and Management
Implementing serialization on a packaging line requires a complex integration of hardware and software and often requires that new and existing systems work together.

PTSM: Pharmaceutical Technology Sourcing and Management
PDA’s new technical report provides a template for bio/pharma companies to follow to establish a risk-based approach to prevent drug shortages.

PTSM: Pharmaceutical Technology Sourcing and Management
Small- to medium-sized companies are expected to drive near-term growth of the global biomanufacturing outsourcing market.

PTSM: Pharmaceutical Technology Sourcing and Management
Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.
PTSM: Pharmaceutical Technology Sourcing and Management
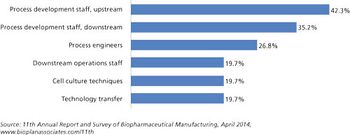
Capacity expansions require staff expansions, and skilled, experienced staff, at all levels, are simply becoming increasingly hard to find.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA has concluded that Kemwell’s facility, systems, and practices comply with FDA’s requirements and no observations were reported on Form 483.

PTSM: Pharmaceutical Technology Sourcing and Management
Recipharm expects to be rolling out further serialization projects during 2015.

PTSM: Pharmaceutical Technology Sourcing and Management
The investments made in analytical and process development equipment will enable Laurus Synthesis to offer chemistry services to its clients.

PTSM: Pharmaceutical Technology Sourcing and Management
UPS expands its special commodities program to more countries.

PTSM: Pharmaceutical Technology Sourcing and Management
Sharon Johnson, senior vice president, Global Quality & Regulatory Affairs, will head the organization.

PTSM: Pharmaceutical Technology Sourcing and Management
The manufacture and scale up production mark a significant milestone in the commercialization route of Nanobiotix lead product, NBTXR3.

PTSM: Pharmaceutical Technology Sourcing and Management
Dr. Reddy’s announced that it expanded its facilities to include a formulation development laboratory at its Miyapur, Hyperabad, India plant.