
John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

Management of the product plan and details for biopharmaceutical production are included in an integrated review of biotechnology operations.

Europe establishes new collaborative system to track products.
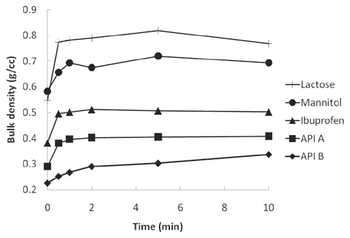
The authors experiment with a resonant acoustic mixer as a method for dry powder coating.

Average deal size and amounts invested increased in the second quarter, but totals trail funding of last year.

AAPS Global Health Focus Group's Kishor M. Wasan discusses new initiatives.
Single-use components in the fill–finish line provides increased flexibility to multiproduct manufacturers.
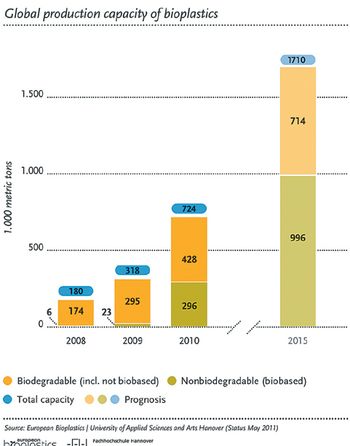
Drugmakers and packagers are pursuing various initiatives to reduce their carbon footprints. This article contains bonus material.

Getting the most value out of M&As requires proper upfront legwork.

Corporate management must be held accountable for quality at all levels.

Expanding the organization's mandate will strengthen inspections.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

Could oral absorption-enhancing technologies change the shape of protein delivery?

The authors describe the concept of the limiting discriminatory the limiting discriminatory threshold (LDT) as an objective means of evaluating the inherent quality requirement of a large-sample content-uniformity test.
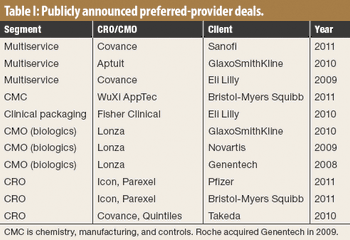
CROs that have made big acquisitions could be outmaneuvered by evolving sourcing models.

The authors summarize a recent FDA–PQRI workshop on process drift.

A Q&A with Rao Tatapudy, vice-president of scientific affairs at Catalent, on recent industry trends.

Letting contamination build up can cause multiple headaches.
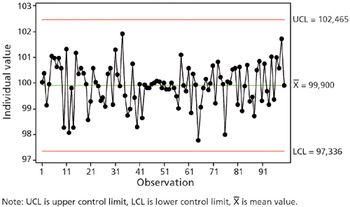
Current GMPs demand full understandng of out-of-control concepts. This article contains bonus material.

In an effort to balance bilateral trade, India is urging China to increase Indian pharmaceutical imports.

New product reviews for October 2011 focusing on analytical instrumentation.

The authors summarize the Matrixx Initiatives, Inc. v. Siracusano case's implications for industry.

Manufacturers fund research and reduce prices to tackle diseases.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Despite new hurdles, industry must move forward and fulfill its mission.

This risk-management case study focuses on assessing empty capsules.

Click the title above to open the Pharmaceutical Technology October 2011 issue in an interactive PDF format.