
The author discusses the regulatory requirements for electronic records contained in Title 21 CFR Part 211 and how they overlap with the requirements set forth in Title 21 CFR Part 11.

The author discusses the regulatory requirements for electronic records contained in Title 21 CFR Part 211 and how they overlap with the requirements set forth in Title 21 CFR Part 11.

Celebrating Pharma Innovation

Holistic open learning networks offer a new drug R&D model for improving research outcomes.
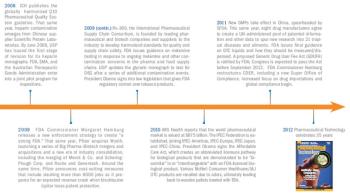
How FDA, USP, and ICH have redirected industry practice.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

The past three decades have driven a purchasing evolution to a procurement revolution.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.

Industry experts share their insight on solid-dosage and sterile manufacturing.

Tracking changes from spinoffs of chemical companies to life-sciences powerhouses.

EMA and MHRA provide insight into increased GMP deficiencies.

IQ Consortium representatives explore industry approaches for applying GMPs in early development.

Q&A with David Elder of Strategic Compliance Consulting, PAREXEL International on preparing for the rise in inspections. Elder is a former senior official with FDA.

Careful attention to detail will help to prevent valuable assets from "melting" away.

A Q&A with Thomas E. D'Ambra, Chairman, CEO, & President of AMRI, on recent industry trends.

Taking time to appreciate the industry's greatest achievements will inspire growth ahead.
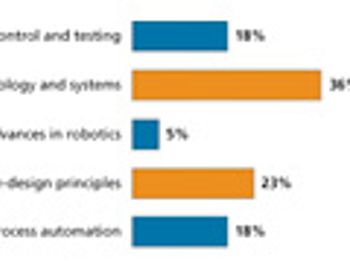
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

A Q&A with FDA Deputy Commissioner Deborah Autor.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Developments in hot-melt extrusion using twin-screw extruders to make solid-dosage drug forms.
New product reviews for July 2012.

Advances in targeted drug delivery and customized release profiles are key goals.

An industry roundtable on how users and makers can best assess and manage black specks.

Improvements in expression platforms and enhanced tools for selecting clones are among the advances of the past few decades.

Applying current principals to traditional factorial designs.

Flow chemistry and microreactors offer alternatives to traditional batch manufacturing.

Uniform dose formulation is key to meeting safety study requirements.

A look back at key nanoformulation advances and what lies ahead for nanoparticle-based drug-delivery systems.

Gold nanoparticles for targeting tumor sites and elastic capsules using nanosized flakes are some recent approaches used to control and target drug delivery.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.