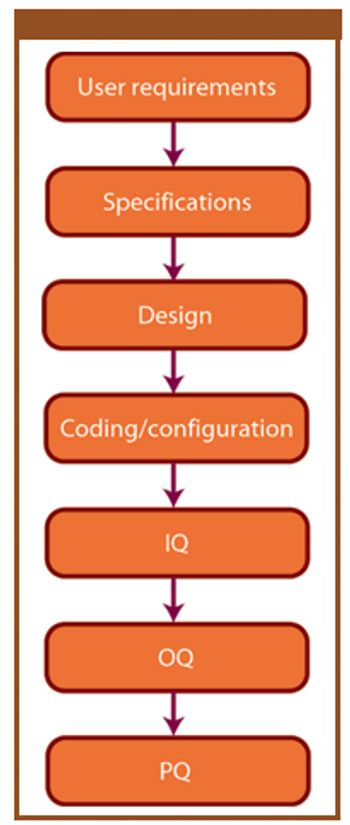
During the past 30 years, manufacturers developed sophisticated packaging and delivery systems to support the requirements of traditional and complex biologics, including quality and cleanliness. This article discusses the evolution of packaging and delivery systems for injectable administration systems as the pharmaceutical and biotechnology industry evolved during the past 30 years. It also explores the future of packaging and delivery systems as technology and drug development advance.