
FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.
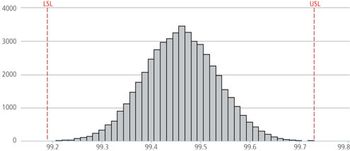
Do you have acceptable control of your instruments and analytical procedures?
Process analytical technology paved the way for continuous manufacturing.

Acquisition binges often lead to hangovers; here’s what to watch out for.

Different types of modular systems have advantages and disadvantages.

Industry experts discuss the benefits and challenges of using single-use systems in pharmaceutical manufacturing.

Application of flow chemistry for small-molecule API synthesis continues to expand thanks to research efforts.

With technology advances, continuous manufacturing shows steady progress to more widespread adoption.

Investment in talent and infrastructure, more quality-by-design skills, and increased communication with global regulators will be needed to combat compliance issues at API and drug manufacturing facilities in India.

Experts discuss the key considerations in the development of an autoinjector.

The authors evaluated the performance and robustness of controlled-release tablets made with HPMC blends of unimodal and bimodal molecular weight distribution.

This article presents recommendations based on a program that was set up to qualify members of a large, diverse team at one oral solid-dosage-form manufacturing facility.

The Cadence Acoustic Separator (CAS) from Pall Life Sciences enables the purification of bioprocesses fluids without the use of primary depth filters or centrifuges.
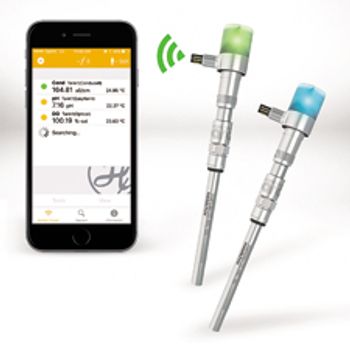
The ArcAir communication package from Hamilton Company enables Bluetooth 4.0 wireless connectivity in all environments.

The MD-Dropper Cap from HealthStar is a new dropper bottle technology for blow-fill-seal (BFS) technology.

Washdown duty ribbon blenders from Ross, Charles & Son can be used to mix dry blends such as grains, powders, pellets, and flakes.

Pharmaceutical Technology spoke with Melissa Topp, director of Global Marketing at ICONICS, about the latest in process analytical technology (PAT).

Click the title above to open the Pharmaceutical Technology June 2016 issue in an interactive PDF format.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on data integrity.