
Excipients Step into the Spotlight

Excipients Step into the Spotlight

The birth of "the pill" and harmonization created a new paradigm for global standards.

CROs and CMOs adapt their business models and capabilities to meet sponsor companies' need to reduce costs and accelerate development time.

The author suggests industry may need a NATO-type organization to even out inspections.

Industry and regulatory experts discuss excipient testing, regulatory expectations, supply-chain challenges, and pricing in this Speakers Roundtable. This article contains bonus online material and podcasts.

The sovereign debt crisis in the EU could greatly change the landscape for contract services.

Puerto Rico, long a mainstay of solid-dosage manufacturing, intensifies efforts in biopharmaceutical manufacturing and research and development.

Interphex attendees found packaging machines and containers with increased functionality. This article contains online bonus material.

FDA and industry seek to ensure drug quality and safety in a world complicated by global outsourcing and rising theft.

The president of Binder, a manufacturer of simulation chambers, addresses the consolidation of Big Pharma, and more.

A PharmTech forum to discuss the industry's efforts in corporate social responsibility and sustainability.

USP membership meeting prepared the standards-setting body to meet modern challenges.

Editors' picks of pharmaceutical science and technology innovations.

A timely new book explains techniques for conformational analysis.

Europe moves to place excipient GMP and GDP standards on the same level as active pharmaceutical ingredients.

Too much or too little control can actually lead to the same result.
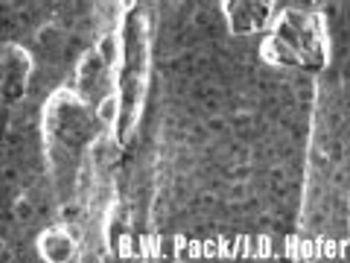
The authors recommend a strategy for classifying similar nonstainless-steel surfaces into three groups based upon the analytical recovery that was observed in this study.