Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.
Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.

With counterfeiting on the rise and Europeans worried their backyard is becoming a base for such illegal activity, legislators have proposed a series of solutions that span the continent and abroad.

Getting IT, engineering, and manufacturing on the same page requires a delicate balance.

The authors survey the approved applications of dimethyl sulfoxide USP, PhEur across the healthcare industry and consider the suitability of DMSO from a regulatory and formulation compatibility standpoint.

Enterprise process control and management (EPCAM) is a new strategy for healthcare manufacturers based on recent process-control breakthroughs in the electronics industry.

Florida is making its case for pharmaceutical and biotechnology research and development.

ISA 100.11a and WirelessHART both seek to become the global standard for industrial wireless automation.

An authoritative book helps drug developers face one of their toughest problems.

The US Food and Drug Administration announced its Pharmaceutical GMPs for the 21st Century initiative six years ago. This article reports on the outcome of a recent workshop on this topic and the action plan set forth.

The less complex nature of excipient manufacturers, as compared with API manufactures, carries many benefits.

Editors' Picks of Pharmaceutical Science & Technology Innovations
Brief pharmaceutical news items for May 2008.
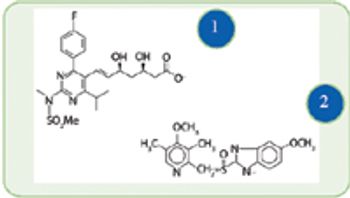
Chemocatalytic and biocatalytic routes show promise for more efficient syntheses of select active ingredients.
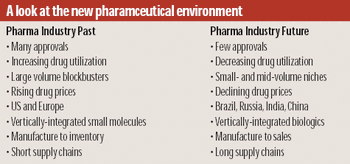
CMO business models are out of touch with current pharma industry realities. It's time for new thinking.

Regulators face demands to improve postmarket surveillance and meet review deadlines.

INTERPHEX 2008 offered visitors novel experiences and many stimulating sessions.

WFI system deficiencies and damp tax records cause problems for two plants

According to the latest figures from IMS Health, Inc., Japan's pharmaceutical market is expected to grow 1–2% this year compared to global industry growth expectations of 5–6%.