
Solid Dosage and Excipients

Solid Dosage and Excipients

Editor's picks of analytical instrumentation products for April 2011.

PRTM helps to improve supply-chain management and logistics in developing countries.

Courts and Congress seek to reshape policies and programs.

An uncertain regulatory environment affects funding for biotechnology.

Research and development may be headed for divorce.

The contract-research industry in China is growing in leaps and bounds, and Big Pharma is leading the way.

FDA reviewers aim to assist ANDA sponsors in building quality into their submissions by clarifying components of the applications. Part 4 addresses manufacture and container closure.

Regulators question whether particles that they can't see hurt patients.

PhRMA efforts of industry's R&D scientists.
Chemocatalytic and biocatalytic routes play an important role in improving the manufacture of intermediates and active pharmaceutical ingredients.

A review of current efforts within PDA's Paradigm Change in Manufacturing Operations initiative.

The authors revisit their previous effort to refine the terms that describe interventions and to dispel confusion that arose after the original article was published.

The complexity of third-party external supply networks requires new ways to manage them.

In any industry, inspections can be a pain, and pharma is no exception.

Companies engaged in global mergers and acquisitions may be hearing from the Department of Justice more often to ensure that corruptive practices are not taking place.
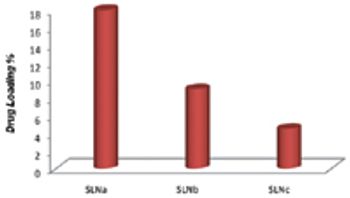
The aim of this study was to prepare and characterize physiochemically and biologically tamoxifen-loaded SLNs to evaluate their effectiveness as a drug-delivery system to treat breast cancers.

The need for greater process understanding raises the bar for suppliers.

The hardest errors to spot are the ones that don't look like errors at all.

Bob Weaver, president of HunterLab, discusses current trends and challenges.