As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Room-temperature sterilization using nitrogen dioxide gas benefits parenteral drugs.

Instrument manufacturers and testing service providers are offering improved tools to biologic charaterization.
The authors present topics discussed and conclusions that resulted from the PDA QbD workshop.

Companies risk drowning in alphabet soup if the latest three-letter acronym improvement strategy isn't clearly linked to business strategy.
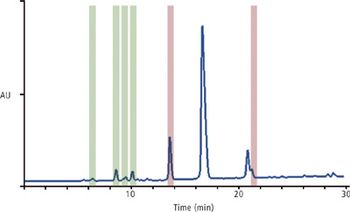
The authors demonstrate that using supercritical fluid chromatography offers distinct advantages in speed and in clean isolation of the desired peaks.

FDA's requirements for API manufacturers in regards to ICH Q7.
Engineering nanoparticles with optimal properties for use in cancer therapies.

Past IPEC-Americas excipient qualification committee chairs highlight changes to the IPEC guide on certificates of analysis for bulk excipients.

Discussions are underway as the pharmaceutical sector calls for greater consistency in the global monitoring of GMP compliance and quality testing of APIs and finished medicines.

A roundup of regulatory news from across the global pharmaceutical industry.

Industry experts discuss the effect FDA's 2011 process validation guidance has had on industry.

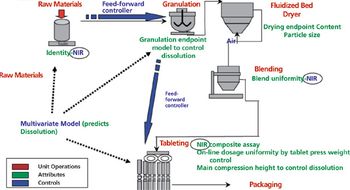
Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

QbD paradigm advances process understanding in development and manufacturing.

New product reviews for March 2013, featuring automation, IT, and process control systems.

Visual mapping can provide a particle-size distribution estimate.

Vaccine development is benefiting from manufacturing advances and support for global health.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.

The authors review developments in wet granulation using a twin-screw extruder.

Brazil's major vaccine producer innovates with stem-cell research.

Click the title above to open the Pharmaceutical Technology March 2013 issue in an interactive PDF format.