
Applying QbD Principles to Drug Substance Development and Manufacture : Inside ICHQ11

Applying QbD Principles to Drug Substance Development and Manufacture : Inside ICHQ11

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Pharma announces plans for the year ahead at annual JPMorgan Global Healthcare conference.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

Guidance offered on how to deal with off-label information requests.

As biopharmaceutical development and commercialization increases, companies are expanding their cold-chain capabilities.

In Part I of a three-part article, the authors discuss what constitutes an impurity and the potential sources of impurities in APIs and finished drug products.
Where is the variability coming from and what have we done to minimize it?
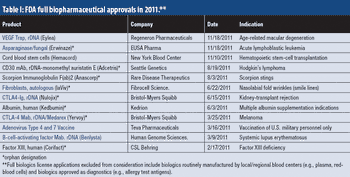
Industry optimism is on the rise for 2012.

A new class of nanoparticles hold promise for preventing premature drug release and offering greater accuracy and effectiveness in drug delivery.

New educational programs are key to the industry's future and to safe, available drugs.

The Asian nation is strategizing to take the lead over its regional competitors in pharmaceutical exports.

New product reviews for February 2012.

Debottlenecking downstream mAb purification.

FDA and industry expert working group representatives discuss the pending ICH Q11 guideline.

ICH Q8, Q9, and Q10 support and implications for the future.

Experts discuss solutions for filter bacterial retention and related challenges. Contains online bonus material.