
Pharmaceutical Technology Europe
With appropriate standardization from an interfacing point of view, separation science will find a broader audience in PAT.

Pharmaceutical Technology Europe
With appropriate standardization from an interfacing point of view, separation science will find a broader audience in PAT.
Pharmaceutical Technology Europe
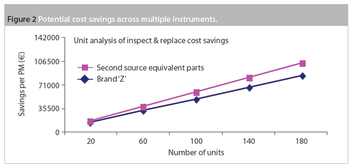
Maintenance and service-related items are often the second-largest budget element in a laboratory after salaries and benefits. Within maintenance, preventive maintenance (PM) is a substantial portion of the budget. Traditionally, PM was an equipment maintenance philosophy based on replacing, overhauling or remanufacturing a piece of equipment at fixed intervals, regardless of its condition at the time. In essence, it involved fixing something that wasn't necessarily broken and this approach is still widely used in the pharmaceutical industry.
Pharmaceutical Technology Europe
Pharmaceutical manufacturers are under increasing pressure to shorten time-to-market, produce treatments with unpredictable product lifetimes, provide greater flexibility and, at the same time, comply with ever more stringent quality, validation, stability and traceability constraints. While this is encouraging for the contract manufacturing sector, it creates the need for even greater manufacturing flexibility.

Pharmaceutical Technology Europe
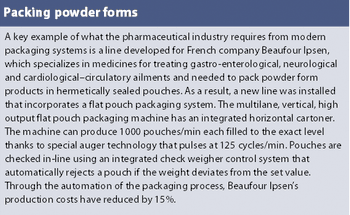
Vaccines are needed against old and new infectious disease threats - polio and other childhood illnesses, bioterrorism and pandemic flu. They are also emerging for cancer immunotherapy and for treating addiction. While vaccines are among some of the most successful biotech products, their large-scale manufacture involves some special demands, such as maintaining a good working cell bank and gearing up for production on an 'as needed' basis.

Pharmaceutical Technology Europe
Some people claim the statins are so effective and that their cholesterol has dropped to a point where they can "supersize" their burger and chips every time they visit a fast food outlet

Pharmaceutical Technology Europe
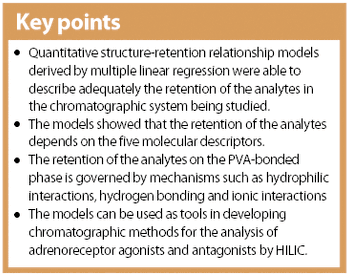
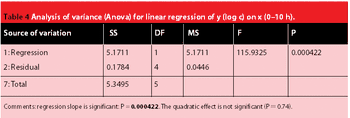
The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.
Pharmaceutical Technology Europe

Pharmaceutical Technology Europe
When the United States Pharmacopeia (USP) announced a delay to the proposed implementation date of July 2007 for General Chapter <467> Residual Solvents, pharmaceutical companies supplying the US market welcomed the interim reprieve. However, meeting the new implementation date will prove challenging as the requirement applies to all existing products covered by the USP and companies must still work hard to prepare for July 2008 when <467> will finally come in.
Pharmaceutical Technology Europe