Pharmaceutical Technology Europe
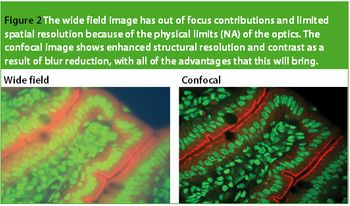
In the biological arena, new and highly useful fluorescent markers are used to stain or 'label' specific structures of interest. They have transformed the range and applicability for optical observation. These labels are excited and correspondingly emit at specific wavelengths; thus, different facets of a specimen can be 'selected' by controlling the wavelength of the delivered and captured light. For example, labels such as 4',6-diamidino-2-phenylindole (DAPI) are used to highlight the nucleus of a cell and MitoTracker Orange is used for mitochondria. Figure 1 shows an example of a multiple stained section, viewed in fluorescence. There has been an explosion in fluorescent labels for examining biological structures, in fixed and live cell preparations.