
Pharmaceutical Technology Europe
Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

Pharmaceutical Technology Europe
Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.


Pharmaceutical Technology Europe
Though many advantages are associated with the European Clinical Trials Directive, complexities have emerged since its introduction in 2004. Since 2007, efforts have been made to raise the issues and address the negative impact of the Directive.

Pharmaceutical Technology Europe
Why spray drying processes offer a range of particle engineering possibilities.

Pharmaceutical Technology Europe
The author assesses the power and limitations of NIR chemical imaging and its future.

Pharmaceutical Technology Europe
Though the pharma industry has improved its change management processes, there are still opportunities for improvement.

Pharmaceutical Technology Europe
Bioanalysis has migrated from a very general vocation to one that requires more specialization, particularly in GLP applications.

Pharmaceutical Technology Europe
Advances in dissolution testing technology have been one of the biggest breakthroughs in solid dosage form testing over the last decade.

Pharmaceutical Technology Europe
The needs of the pharmaceutical industry for powder testing technologies are changing, primarily for two reasons.

Pharmaceutical Technology Europe
Over the last few months, we have been publishing a series of special features, which have sought to provide you with an overall view of current challenges, innovations and trends in niche pockets of the pharmaceutical industry.

Pharmaceutical Technology Europe
The emerging markets represent an attractive investment opportunity for the pharmaceutical industry because of the countries' growing economies and unmet medical needs.
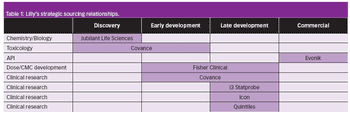
Pharmaceutical Technology Europe
Lilly faces up to the new realities of the bio/pharmaceutical industry by embracing outsourcing.

Pharmaceutical Technology Europe
The pharmaceutical industry must understand its responsibilities to improve the safety of chemicals as defined by the REACH initiative.