
Pharmaceutical Technology Europe
No one is better than Europe when it comes to innovative products at the forefront of the industry

Pharmaceutical Technology Europe
No one is better than Europe when it comes to innovative products at the forefront of the industry
Pharmaceutical Technology Europe
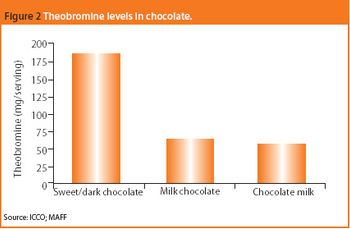
Could compounds in chocolate yield new pharmaceutical approaches to major disorders?

Pharmaceutical Technology Europe
All sectors of manufacturing are under continual pressure to bring new products to market quicker, stealing a march on the competition and maintaining their revenue stream.

Pharmaceutical Technology Europe
The right choice [of coding and marking technology] depends upon the company's top priorities regarding legibility, cost, speed, ease of use, cleanliness and security.
Pharmaceutical Technology Europe
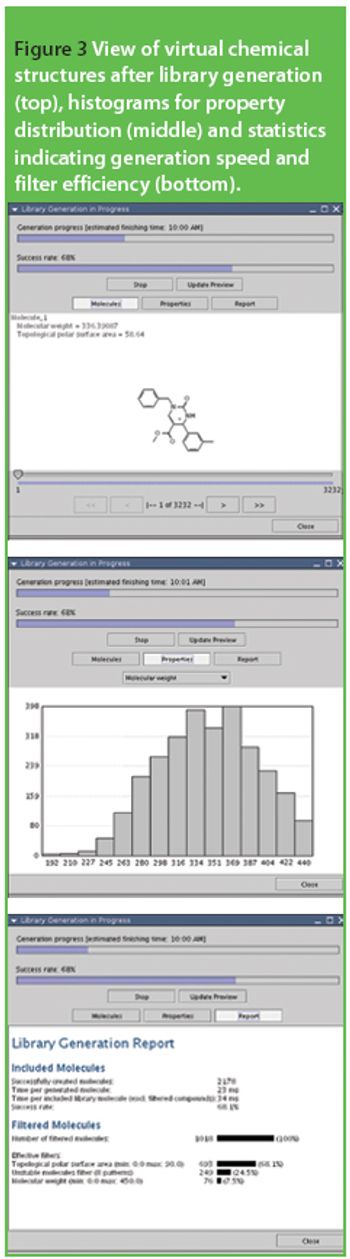
The discovery of suitable lead structures for new drugs from an inexhaustibly large reservoir of theoretically possible compounds is one of the biggest challenges for the pharmaceutical industry. In the last few years, combinatorial chemistry methods have been developed to synthesize a huge amount of diverse new chemical entities (NCEs), which may subsequently be tested for biological activity in vitro.
Pharmaceutical Technology Europe
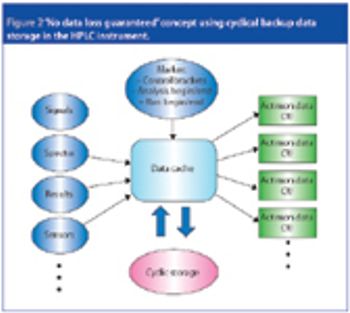
The latest generation of HPLC instruments offers a new level of data security. Improvements in embedded instrument control programs and mass storage functionality offer 'no data loss' guarantee. This level 5 instrument control makes data acquisition audit-safe and increases laboratory technicians' efficiency, freeing them up from any reanalysis work.
Pharmaceutical Technology Europe
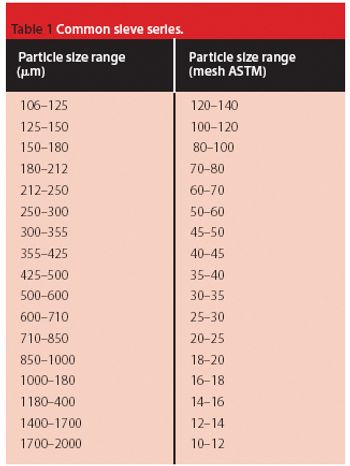
Pellets are a multiparticle, solid form of medication. The individual pellets are almost spherical with diameters usually between 100 and 2000 ?m.

Pharmaceutical Technology Europe
Creation and qualification of scale-down models are essential for performing several critical activities that support process validation and commercial manufacturing. As shown in Figure 1, these activities include process characterization and production support studies that are performed to evaluate column and membrane lifetimes, demonstrate clearance of host-cell impurities and viruses and troubleshoot manufacturing issues. While the underlying fundamentals are relatively the same as those when scaling up, some unique considerations should be taken when scaling unit operations down.4

Pharmaceutical Technology Europe
The pharmaceutical sector is a billion dollar industry with a huge responsibility towards its customers and investors. The main tools for fulfilment of this responsibility are ensurance of compliance and maintenance of control. It is a time-consuming job to uphold these responsibilities, and many important decisions regarding this subject are taken every day. It is important to make carefully considered decisions and follow them up. It is also essential to stop once in a while and reconsider their validity and relevance.

Pharmaceutical Technology Europe
Once the brightest students are interested in bioprocessing, it is vital that they are prepared and inducted into industry.