
Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...
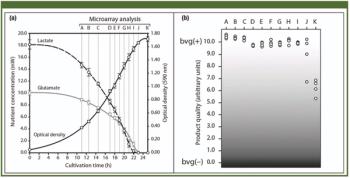
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

Pan coating has been the preferred method of coating tablets for more than 20 years, but core coating is becoming more popular.

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.
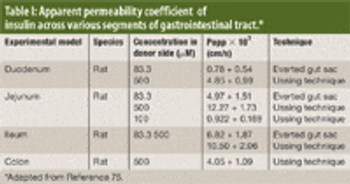
The authors review various oral drug delivery systems that have been explored to increase patient compliance for insulin.
The authors discuss how strategic outsourcing to contract manufacturing organizations that have technical and regulatory expertise can add further value during vaccine development.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

The Physician Payments Sunshine Act is still pending before the US Senate Committee on Finance, according to Jill Kozeny, communications director for Senator Chuck Grassley (R-IA).

Following the 2008 launch of the European Fine Chemicals Group (EFCG) Voluntary Guidelines (VGs) to promote supply-chain security, the group has now announced the official launch of an assessment template that will allow fine chemicals customers and suppliers to assess and implement this new set of recommendations.

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

Following the World Health Organization's declaration last week of an influenza pandemic, vaccine makers in Europe and China as well as world health agencies are stepping up efforts toward rapid development and approval of an effective vaccine.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Bullard and Teledyne Tekmar.

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...
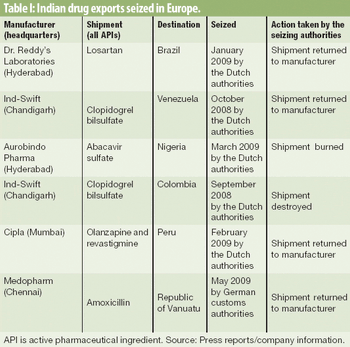
Patent infringement claims and a lack of clear global trade distribution routes may be unraveling the country's generic-drug export industry.

The US Government Accountability Office recommended that the US Food and Drug Administration draft a plan, including milestones, for developing its Sentinel system and ensuring the privacy and security of patients' healthcare data.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...

This week the US Food and Drug Administration released the final version of Guidance for Industry: Providing Regulatory Submissions in Electronic Format-Drug Establishment Registration and Drug Listing.

In two whistleblower suits filed in May 2009, the United States and 16 states alleged that Wyeth (Madison, NJ) knowingly failed to offer the government the same discounts it gave to private purchasers of its drugs, as Medicaid laws require.

The latest growth forecast for the 2009 pharmaceutical market might have come as a shock to many, but there is still hope for the industry in the form of new product launches and potential blockbusters.

The United States Department of Health and Human Services (HHS) this week placed an initial order with Sanofi Pasteur (Lyon, France) for a vaccine to fight influenza A (H1N1) infection.

Novartis (Basel) signed an agreement that grants the company an exclusive option to acquire Elixir Pharmaceuticals (Cambridge, MA) upon the successful completion of a Phase IIa clinical study of Elixir's lead oral ghrelin antagonist, which is now the subject of preclinical studies.

Also, Johnson & Johnson acquires Cougar Biotechnology; NIH launches program for rare and neglected diseases; PPD restructures leadership positions; more...

Pfizer's Pharmacia unit may be ordered to pay nearly $212 million as a result of a February 2009 Wisconsin court ruling that found the company guilty of violating the state's Medicaid fraud statute 1.44 million times.

Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...

Last Monday, Billy Tauzin, president and CEO of the Pharmaceutical Research and Manufacturers of America (PhRMA), voiced the group's support for President Obama's efforts to reform the nation's healthcare system.

The US Food and Drug Administration intends to automate and enhance the safety of the pharmaceutical supply chain by establishing electronic pedigrees (ePedigrees) for drug products.

Automation has been part of pharmaceutical processing for some time, but robotics have not yet found wide acceptance among drugmakers.