
On Sunday, Nov. 8, 2009, the Pharmaceutical Research and Manufacturers of America (PhRMA) expressed its disappointment with the US House of Representatives's healthcare-reform bill.

On Sunday, Nov. 8, 2009, the Pharmaceutical Research and Manufacturers of America (PhRMA) expressed its disappointment with the US House of Representatives's healthcare-reform bill.

The American Association of Pharmaceutical Scientists (AAPS) recognized researchers at the organization's 2009 Annual Meeting and Exposition in Los Angeles this week.

Also, Commissioner Hamburg addresses the nation's doctors in a letter about the influzenza A (H1N1) virus and vaccine.

Also, FDA and WebMD increase collaboration and PPD is awarded contract to evaluate the agency's postmarket spontaneous-adverse-event surveillance system.

In response to a request from the US Food and Drug Administration, the US Pharmacopeial Convention (USP) revised its standards for propylene glycol and sorbitol solution

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.
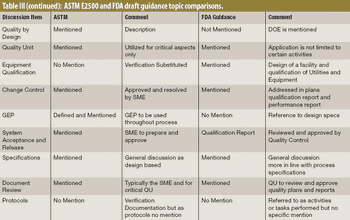
The author explores differences between two qualification documents, the draft guidance from FDA "Process Validation: General Principles and Practice" and the ASTM E2500-7 standard "Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment."

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.

Also, FDA's DDMAC issues Warning Letter to King Pharmaceuticals; draft guidance released for SPL standards for content of labeling.

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

The US Food and Drug Administration is using prescription data from Wolters Kluwer Pharma Solutions to track the treatment of influenza A (H1N1) and other influenza viruses, according to a statement that the company released last week.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

The two agencies issued a joint Warning Letter to the owners of a website selling unapproved products intended to treat the swine flu virus.

The manufacturing process, which influences a drug's safety and efficacy, is particularly critical for drugs administered through injection, and personnel must closely supervise lyophilization to ensure product quality.
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the October 2009 edition from Emerson and Minitab.

Economic pressures are forcing pharmaceutical manufacturers to look for ways to become more competitive. Many companies realize that they must reduce operating costs but maintain or improve product quality.

For the first time, cancer treatments accounted for 5% of overall drug spending in the first half of 2009, said Medco Health Solutions in a press release last week.

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...

Also, USAID and USP launch counterfeiting public service campaign in Cambodia.

Also, FDA partners with USDA on produce safety, and the Transparency Task Force announces its second public meeting...

The US Food and Drug Administration published its first draft guidance for industry about Risk Evaluation and Mitigation Strategies (REMS) on Sept. 30, 2009.

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...

AAPS President offers hope and solutions for the industry's challenging future.

This article introduces the "Q.U.E.S.T." approach for vendor qualification, a practical and compliant methodology for pharmaceutical and biopharmaceutical companies to qualify vendors and hence make well-informed purchasing-related decisions.

The author describes the framework needed to implement QbD and achieve the deeper process understanding that is fundamental to QbD.

The author analyzes, from an agency perspective, whether question-based review has improved product quality or made the review process easier for regulators or for industry.

How manufacturers can accelerate cashflow by cutting out the paper trail.

Also, FDA bans candy and fruit-flavored cigarettes, EMEA moves to improve information management.

Company and People Notes: Boehringer Ingelheim will acquire Wyeth's animal health business; Amsterdam Molecular Therapeutics appoints CEO; more...

Rene Kummer of Sigpack Systems speaks about the company's new pharma toploader, which features an innovative box transportation unit.