Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

The pharmaceutical industry has a history of developing innovative medicines and novel drug-delivery systems, but not all of its manufacturing processes use cutting-edge technologies. Demands to increase productivity in fill–finish lines are fueling the arguments of proponents of robotic automation to expedite manufacturing.

I work in a quality control laboratory and use a pH meter with a single-junction reference probe to test samples taken from various pharmaceutical (drug) production lines. The probe I use has a six-month warranty. It slowly stops working after just a couple weeks. I am replacing it once a month. We make sure to store it correctly according to the manufacturer's specifications and even use probe cleaner at the end of each shift to ensure that it stays clean. What can I do to increase the lifespan to at least cover the warranty period?

Xoma Restructures; AAIPharma Appoints Eric Evans as CFO; and More.

Novartis Consumer Health has announced a voluntary recall of all lots of select, bottle-packaged configurations of Excedrin, NoDoz, Bufferin and Gas-X Prevention. The recall follows consumer complaints of chipped or broken pills and inconsistent bottle packaging line clearance practices that could lead to stray tablets, capsules or caplets from other Novartis products.

USP Releases Guidelines on Ensuring the Integrity of the Pharmaceutical Supply Chain.

AMRI Restructures; ISPE Appoints Nancy S. Berg as CEO; and More.

AstraZeneca Acquires Chinese Generic-Drug Company; Takeda Makes Management Changes; and More.

On Dec. 21, 2011, Ranbaxy Laboratories signed a consent decree with FDA and pledged that it would strengthen procedures and policies to ensure data integrity and to comply with current good manufacturing practices. The agreement is subject to approval by the US District Court for the District of Maryland.

Last week, Baxter International and Momenta Pharmaceuticals entered into a global collaboration to develop and commercialize follow-on biologic products. The two companies expect to close the transaction during the first quarter of 2012, subject to customary closing conditions.

Boehringer Ingelheim Expands Manufacturing Presence in China; Amgen CEO Kevin W. Sharer Announces Retirement Plans; and More.

Last week, the US Department of Health and Human Services and Novartis Vaccines and Diagnostics dedicated a manufacturing plant that can create influenza vaccine using cultured animal cells instead of the conventional expression system of fertilized eggs.

We have several tablet formulations that are dwell-sensitive-they require more time under compression than other formulations. Given increasing demand, we do not have the luxury of slowing the tablet presses down in an effort to increase that dwell time. How can we maximize dwell time and maintain or increase output in our tablet presses?
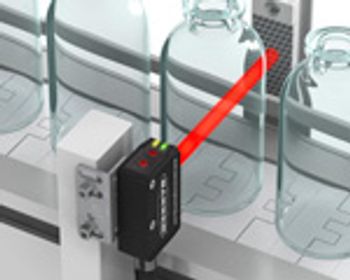
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the December 2011 edition from Banner Engineering and Eriez.
Sterilization or sanitization is usually applied to kill bacteria in a system. Equipment is cleaned to remove residues from the previous batch of product, and subsequently flushed to remove the cleaning liquids. To ensure that sterilization and cleaning are efficient and safe, it is not enough to develop the appropriate procedures. Selecting the right manufacturing equipment further improves cost efficiency, as well as patient safety.

Risk assessment is not a new concept to the pharmaceutical industry, but lately the phrase has become a mantra. A systemic, science-based way to manage risk is becoming essential to meeting the spirit and letter of FDA requirements.

In a Warning Letter, FDA cited "significant violations" of CGMP regulations, including several repeat observations, at three Novartis facilities located in Colorado, North Carolina, and Canada.

The FDA and EMA are moving from "confidence-building to reliance upon" each other in a step-up in cooperation on GMP inspections; the latest move following successful completion of pilot projects this summer.

Baxter International Agrees to Acquire Synovis Life Technologies; Pfizer Elects President and CEO Ian Read as Chairman of Board of Directors; and More.

Merck Establishes New MSD R&D Asia Headquarters; Astellas Adds Two Senior Executives at Agensys; and More.

Last week, the Pharmaceutical Research and Manufacturers of America Foundation awarded Johns Hopkins University and the University of Washington each a $250,000 grant to establish a three-year graduate certificate program. The program is formally known as the PhRMA Foundation Center of Excellence for a Comparative-Effectiveness Research Educational Program. The funds are the foundation's first grants to educational institutions.

In an initiative that could signal a new era in private–public partnerships, AstraZeneca will make 22 compounds available for free to medical researchers next year for projects funded by the United Kingdom's Medical Research Council.

BioMarin Announces FDA Approval of Expanded Biologics Manufacturing Facility; Par Pharmaceuticals Makes Executive Appointments; and More.

EMA Hosts Subgroup Analysis Workshop.

On Nov. 21, 2011, Gilead Sciences agreed to acquire Pharmasset for $137 per share in cash, or a total of approximately $11 billion. Pharmasset's board of directors unanimously approved the transaction, which is expected to close during the first quarter of 2012.

On Nov. 19, 2011, Ben Venue Laboratories voluntarily and temporarily suspended the manufacture and distribution of products made at its Bedford, Ohio, facility. These products include Doxil (doxorubicin HCl liposome injection), which the company produces for Johnson & Johnson. The company's clients include Pfizer, Hospira, and Teva.

FDA Holds Special Press Briefing Regarding Revocation of Genentech's Avastin.

Pfizer, MIT Break Ground on New Research Units in Massachusetts; PhRMA Names Richard I. Smith Executive Vice-President of Policy and Research; and More.

World Diabetes Day on Nov. 14, 2011, saw the release of the International Diabetic Federation's (IDF) fifth edition of the Diabetes Atlas, which produced some staggering statistics for future escalation of the disease.

Ablynx, Merck Sorono Expand Osteoarthritis Partnership; Genzyme Makes Senior Appointments to Multiple Sclerosis, Rare Diseases Businesses; and More.

At a press conference held Nov. 10, 2011, FDA Commissioner Margaret Hamburg said the agency had approved 35 novel medicines in fiscal year 2011. Among the approved products were two drugs for late-stage metastatic melanoma and the first drug to treat Hodgkins lymphoma in 30 years.


