
AstraZeneca to Acquire Ardea Biosciences; Pfizer to Divest Nutrition Business to Nestlé; and More.

AstraZeneca to Acquire Ardea Biosciences; Pfizer to Divest Nutrition Business to Nestlé; and More.

Amgen to Acquire KAI Pharmaceuticals; BASF, Catalent Enter into Bioavailability Collaboration; and More.

Amgen to Acquire KAI Pharmaceuticals; BASF, Catalent Enter into Bioavailability Collaboration; and More.

The management board of the European Medicines Agency has introduced a range of new measures to strengthen and extend its conflicts of interest policy for scientific-committee members and experts, as well as for members of the management board.

Amgen, AstraZeneca Agree to Codevelop Monoclonal Antibodies; Fujifilm Diosynth Biotechnologies Expands R&D Capabilities at its UK Site; and More.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations

Catalent Completes UK Expansion; Novasep Opens New Facility in China; and More.

FDA Issues Warning Letter to Warner Chilcott; Sigma-Aldrich Expands in Asia; and More.

Excellence United, an alliance of six specialty equipment companies, will make its first North American appearance at INTERPHEX 2012, held May 1–3 at the Javits Center in New York.

AstraZeneca Files Suit Against FDA; Pfizer, Biocon Terminate Biosimilar Alliance; and More.

BASi Restructures; Celerion, Ricerca Biosciences Form Biosimilars Alliance; and More.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other private and public organizations.

JHS Secures Four Sterile Parenteral Products Manufacturing Contracts; Samsung Biologics, Biogen Idec Establish Biosimilars Joint Venture; and More.

Biogen Idec to Acquire Stromedix; Mylan, Pfizer Announce Epinephrine Autoinjector Settlement Agreement; and More.

EMA Releases Genetic Variability Guideline.

Alexion Completes Acquisition of Enobia Pharma; BMS Completes Acquisition of Inhibitex; and More.

We need to measure the particle size of a moisture-sensitive powder. We would like to use dry analysis on our laser-diffraction system, but the particles are fragile and we're struggling to get robust results. Is wet measurement with a nonaqueous solvent the only option?

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the February 2012 edition from ACS Valves and G-Con.

Following a packaging error affecting birth-control pills, Pfizer has issued a one-million pack recall and warned women in the US who have been taking the medication during the last several months to consult with their physicians.

FDA Addresses the Regulation and Approval Process of PET Drugs.

AstraZeneca Restructures; Catalent Completes Expansion in Italy; and More.

The Asian nation is strategizing to take the lead over its regional competitors in pharmaceutical exports.

Amgen Agrees to Micromet for $1.16 Billion; Cephalon Issues Recall of Treanda; and More.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other private and public organizations.

2012 Facility of the Year Awards are Announced; Suzhou Pharma Services Names Oliver Mueller President; and More.

The EMA has released a concept paper for a guideline on pharmacogenomic methodologies in the evaluation of authorized medicines to address the fact that genetic differences can cause variability in drug therapy efficacy and safety.

Constellation Pharmaceuticals announced that it has entered into a broad collaboration with Genentech, a member of the Roche group, to discover and develop innovative treatments based on epigenetics, which focuses on the identification of small molecule inhibitors of protein activity.

Takeda Restructures; Savient Pharmaceuticals Names David Veitch President of Savient Europe; and More.
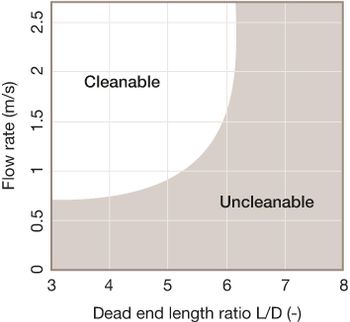
Understanding equipment design and product-contact surfaces enables strong cleaning action.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the January 2012 edition from ACS Valves and Terra Universal.