
Maryland is the latest state to consider whether to include additional requirements for substitution of biological products
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Maryland is the latest state to consider whether to include additional requirements for substitution of biological products

As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Company considers investment in insulin cartridge-filling and insulin API manufacturing capacity in the US.

Company issues voluntary recall after learning of complaints of an uncharacteristic odor coming from Levoxyl bottles.

As more companies try to ensure that their validation activities are compliant and cost-effective, it has become increasingly important for quality professionals and validation technicians to manage costs and reduce downtime by accurately evaluating their validation equipment needs

The Access to Medicines Index shows that pharmaceutical companies are increasing drug development for the developing world and using more tiered pricing mechanisms to lower pricing.

Ruling has implications for intellectual property protection for innovator drugs in India.

Restructuring and a strategic focus on three hubs in research and development are part of a plan to restart growth.
Continuous flow chemistry offers potential for greater control, improved safety and environmental profiles, and efficient chemical transformations.

Contract API manufacturers proceed with select investment in capacity and service additions.

Novartis loses an appeal in seeking patent protection in India for its anticancer drug.

Incremental innovation advances medicines by expanding therapeutic classes, increasing the number of available dosing options, discovering new physiological interactions of known medicines, and improving other properties of existing medicines

Contract API manufacturers and fine-chemical producers proceed with select investment in capacity and service additions.
Supply-demand fundamentals show strong growth for generic APIs as gains in innovator APIs lag.

Biopharmaceutical production is an often discussed application for single-use technologies, but single-use technologies also have application for small-scale finished drug-product manufacturing for producing clinical-trial materials.
As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Instrument manufacturers and testing service providers are offering improved tools to biologic charaterization.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

Factors for assessing excipient variability, the associated challenges developers need to address to design and manufacture solid oral drug products, and solutions for such challenges are examined.

As fine-chemical producers, custom manufacturers, and pharmaceutical companies gathered this week for Informex in Anaheim, California, one observation stands out: for all the inroads that biologic-based drugs have made, the pharmaceutical industry remains a small-molecule marketplace.

As contract API manufacturers, fine-chemical producers, and pharmaceutical companies prepare to attend Informex in Anaheim this month, the state of the industry shows improving conditions in certain sectors.

Heated magnetic nanoparticles consisting of a liposome nanocontainer with superparamagnetic iron oxide nanoparticles are among the recent advances in nano-based drug delivery.

Pharma companies intensify R&D efforts to address treatments for neglected tropical diseases in the developing world.

In the era of manufacturing capacity rationalizaton, tighter return on assets, and re-alignment of manufacturing assets to meet changing product demand, strategies for cost-effectively managing manufacturing and other facilities become ever-more crucial. A Q&A with UMS Advisory.
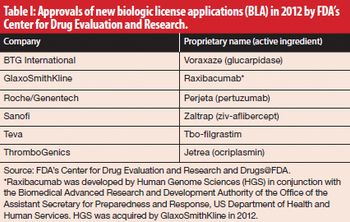
The share of biologic-based drugs in the global pharmaceutical market is on the rise.
Researchers use inorganic catalysts as an alternative to biocatalysts in the selective conversion of sugars to produce chiral building blocks.

CROs are keeping pace with the increased globalization of the biopharmaceutical/pharmaceutical industry through a combination of acquisitions, partnerships, and select investments.

In the arena of pharmaceutical outsourcing, when speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.

When speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.

Financing from initial public offerings and global mergers and acquisitions were down in 2012 and venture-capital funding stayed strong.