
An initiative to develop consensus-based industry standards to identify and define green chemicals and process technologies is underway.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

An initiative to develop consensus-based industry standards to identify and define green chemicals and process technologies is underway.

US Food and Drug Administration Commissioner Margaret Hamburg outlined last week six initial steps designed to improve the effectiveness and timeliness of FDA's regulatory and enforcement system.

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

A joint biopharmaceutical manufacturing facility in India by Kemwell and Boehringer Ingelheim ushers in a new era.

Collaborative planning and execution among clinical research, clinical operations, and supply-chain managers are key elements in effectively managing an increasingly complex and global supply chain for clinical trial materials.

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported in late July that Eurostat, the Statistical Office of the European Communities, recently published a baseline study or the first snapshot of REACH policy in the preregistration phase.
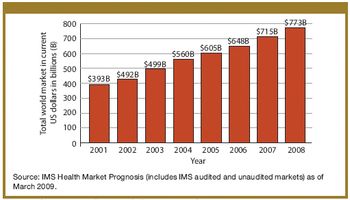
Biologics enhance their positions amidst slowing growth in the global and US markets.

In the aftermath of recent restructuring, Big Pharma is sporting a reduced global manufacturing footprint while intensifying its focus to biologics and emerging markets. What will be the look of tomorrow's manufacturing networks?

Leading experts share their perspective on the specialized requirments when developing a pediatric formulation and examine dosage forms that can be used for this patient class in this roundtable moderated by Patricia Van Arnum.

Leading contract manufacturing organizations share their views on the current and future market dynamics shaping pharmaceutical outsourcing.

In a move to strengthen development of its biologics portfolio, Bristol-Myers Squibb (BMS, New York) has agreed to acquire the biopharmaceutical company Medarex (Princeton, NJ) for $16 per share or approximately $2.4 billion.

The Society of Chemical Manufacturers and Affiliates (SOCMA) this week stated its support to the US Senate for approving legislation that would extend existing chemical security standards for one more year.

Johnson & Johnson has agreed to pay $1.0 billion to acquire the assets and rights of the Alzheimer's immunotherapy program of the biopharmaceutical company Elan, form a new company with Elan based on the AIP program, and gain an 18.4% stake in Elan.

As the pharmaceutical industry turns its efforts to biologic-based drugs and to more targeted delivery approaches, new tools are needed. Some recent developments are discussed.

Advances in micellar catalysis, solid-state chemistry, catalytic asymmetric synthesis, and function-oriented synthesis for natural products represent noteworthy developments in green chemistry that can be applied to the synthesis of active pharmaceutical ingredients.

A joint biopharmaceutical manufacturing facility in India by Kenwell and Boehringer Ingelheim ushers in new era.

The Federal Trade Commission (FTC) last week issued an interim report that examined the effects of authorized generics on competition in the prescription drug market.
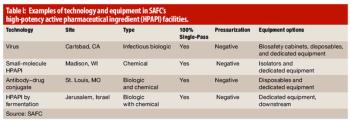
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Generic-drug and specialty pharmaceutical manufacturer Watson Pharmaceuticals (Corona, CA) agreed to acquire the generic-drug company Arrow Group for $1.75 billion in cash and stock.

The Subcommittee on Health of the US House of Representatives Energy and Commerce Committee held hearings last week to discuss the findings of a report by the Federal Trade Commission (FTC) that examined the competitive effects for follow-on-biologics (FOBs).

Kemwell (Bangalore, Karnataka, India) plans to build a new biopharmaceutical manufacturing plant in Bangalore, India, in a strategic collaboration with Boehringer Ingelheim (BI, Ingelheim, Germany), according to a Kemwell press release.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

The US biotechnology industry reached aggregate profitability for the first time ever in 2008, representing the only bright spot in an otherwise dismal year for biotechnology financing and performance.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

Members of the House Committee on Energy and Commerce, Chair Emeritus John D. Dingell (D-MI), Chair Henry A. Waxman (D-CA), and Reps. Frank Pallone (D-NJ), Bart Stupak (D-MI), Diana DeGette (D-CO) and Betty Sutton (D-OH) released last week a discussion draft of the Food Safety Enhancement Act of 2009.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.

Archers Daniel Midland Company (Decatur, IL) is bringing on line a new propylene glycol plant in Decatur, Illinois, in the fourth quarter of 2009.

The World Health Organization's Ad Hoc Policy Advisory Working Group on Influenza A (H1N1) Vaccines issued an update on May 18, 2009, on the production status of seasonal and A (H1NI) vaccines.

Sanofi-aventis announced this week that it plans to construct a new vaccine-manufacturing center in Neuville-sur-Saône, France.

Sanofi-aventis introduced this week its Biolaunch project at its Vitry-sur-Seine, France, pharmaceutical production site.