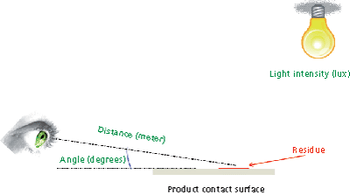
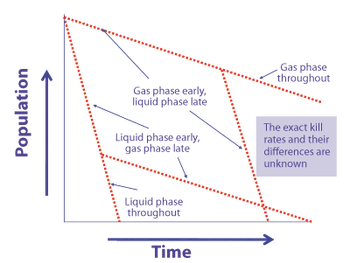
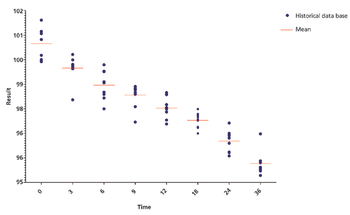
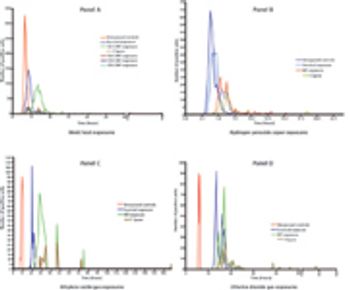
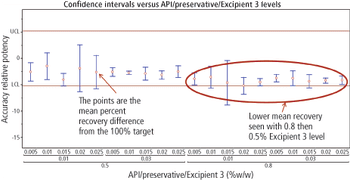
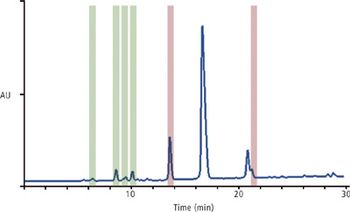
Predicting long-term storage stability of a given protein and formulation is desirable for effective screening and optimization early in the development process. Multiple routes to aggregation during storage to suggest that multiple measurement types should be made to probe different aspects of protein behavior.