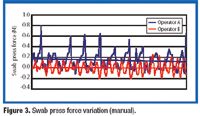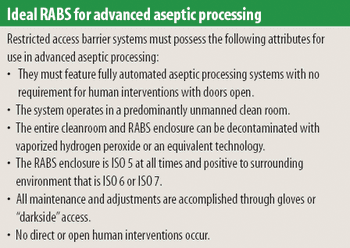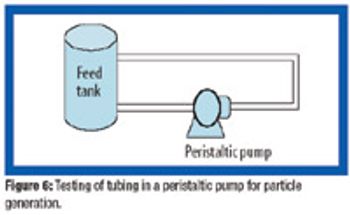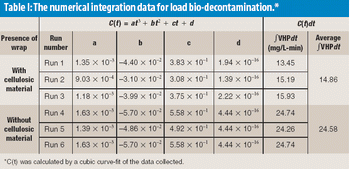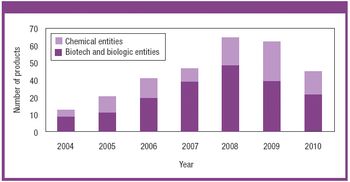
There is a growing need for patient-compliant dosage forms within the cancer therapeutics and biotechnology areas. Ease of administration, enhanced therapeutic efficacy, and reduced side effects are factors that differentiate drug delivery products from conventional dosage forms and provide a competitive advantage. This article reviews salient trends in the parenteral drug delivery sector within the realms of a changing regulatory environment, drivers to growth, and recent advances in this field. Challenges associated with bringing parenteral drug delivery concepts to commercialization are discussed.