
New advanced aseptic manufacturing technologies are available for filling liquid pharmaceuticals, including biologics.

New advanced aseptic manufacturing technologies are available for filling liquid pharmaceuticals, including biologics.

Funding boosts life-sciences manufacturing in West Michigan.

The effect of absorbed vapor-phase hydrogen peroxide on a lyophilized product Protein Z, was studied by spiking experiments with different amounts of hydrogen peroxide.

Application of single-use technology in a parenteral facility for syringe filling.

Catalent's Advasept platform uses blow-fill-seal technology to aseptically manufacture, fill, and seal a polymeric primary container for injectable drugs.

The ionHP biodecontamination hydrogen peroxide-based sterilization technology is designed for use in aseptic enclosures.
Common challenges and key considerations when developing a freeze-drying cycle for protein pharmaceuticals.

Dalton Pharma completes its $2.5 million expansion in Toronto for sterile and aseptic filling capabilities.

A new process analytical technology based on impedance spectroscopy has potential applications for characterizing product attributes during the freeze-drying process.

Changes in the working procedures and disposable fluid flow paths resulted in a measurable decrease in product waste in a low-volume, high-value fill-finish line.

Progress in delivery science, manufacturing technologies, and commercialization are playing critical roles in advancing the development of complex parenteral drug formulations for new drug substances having a variety of formulation challenges

The Parenteral Drug Association has established a task force to develop a peer- and regulatory agency-reviewed Technical Report that will serve as a science-based industry reference document.

Recipharm has invested EUR 32 million ($43 million) in its Wasserburg, Germany site to expand lyophilization capacity.

Aesica expands aseptic capabilities at its Nottingham, UK site with the acquisition of new pre-filled syringes manufacturing equipment.

After realizing benefits from the adoption of a single-use filling line for one specific product, Patheon took the leap and installed a single-use filling line in a new facility.

A recent market analysis offers a promising outlook for contract fill-finish and lyophilization services.

CentuRecon enables dry formulations of therapeutic proteins to quickly be prepared for injection at high concentration.

RABS is a flexible barrier system that maximizes product control but minimizes operator interaction when best practices are followed.

Susan Schniepp, vice-president of quality and regulatory affairs at Allergy Laboratories and co-chair of the program planning committee for the 2013 PDA/FDA Joint Regulatory Conference, discusses quality systems and related considerations in parenteral drug manufacturing.

New cartridges designed specifically for use on high-speed filling lines increase efficiency.

Report outlines recommended practices for control and evaluation of operations.
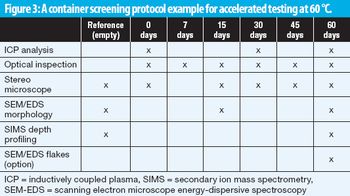
A screening method aligned with USP 1660 guidance predicts glass delamination in primary packaging for parenterals.

The benefits of single-use systems are being realized for downstream unit operations, including aseptic filling.

Aseptic connectors provide the flexibility and robustness needed for modern parenteral manufacturing operations.

Adoption of single-use systems and more flexible systems drive innovation.