
Ilya Pharma has been granted approval by the Swedish Medical Product Agency and Swedish Ethical Review Authority for initiation of a Phase II clinical trial of its ILP100 gene therapy.

Ilya Pharma has been granted approval by the Swedish Medical Product Agency and Swedish Ethical Review Authority for initiation of a Phase II clinical trial of its ILP100 gene therapy.

Horiba has joined a consortium aimed at accelerating the development of process analytical technologies for cell and gene therapy manufacturing

Yposkesi is building its second commercial facility for cell and gene therapy manufacturing at its campus on Corbeil-Essonnes, France.

The CGT Catapult has formed a consortium aimed at advancing the technology development and lowering costs of cell and gene therapy manufacturing.

VIVEbiotech has opened its new lentiviral vector manufacturing facilities in Spain, expanding capacity for lentiviral vectors for use in cell and gene therapies.

The Discovery Labs has signed a foundational lease with the University of Pennsylvania Gene Therapy Program to build a new life sciences cluster in King of Prussia, Pa.

Biogen and Ginkgo Bioworks have partnered to develop a next-generation AAV production platform to accelerate Biogen’s gene therapy drug development efforts.

The Committee for Medicinal Products for Human Use of the European Medicines Agency presented a positive opinion of bluebird bio’s SKYSONA (elivaldogene autotemcel, Lenti-D), a one-time gene therapy for the treatment of early cerebral adrenoleukodystrophy, an X-linked metabolic disorder.

Univercells will introduce the intermediate capacity scale-X carbo system to NIBRT’s facility for hands-on demonstration and training in CGT.

VectorY, a Netherlands-based biotechnology company, has been selected as the European winner of MilliporeSigma’s 2021 Advance Biotech Grant Program.

Charles River plans to enhance its gene therapy capabilities through the acquisition of Vigene Biosciences.
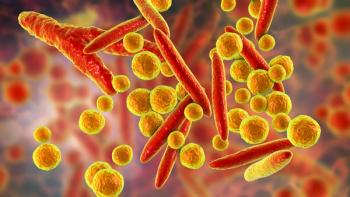
The sensitivity of droplet digital PCR helps manufacturers detect Mycoplasma contamination in AAV-based gene therapy products.

Biogen and Capsigen have entered into a strategic research collaboration to engineer novel AAV capsids to deliver gene therapies that address the underlying genetic causes of various central nervous system and neuromuscular disorders.

Originally established in the 1970s as a fetal bovine serum supplier, Fujifilm Irvine Scientific continuously proved its strength in the cell culture solutions space.

NIH reported that an investigational gene therapy developed by researchers from the University of California, Los Angeles and Great Ormond Street Hospital in London can restore the immune systems of infants and children who have severe combined immunodeficiency due to adenosine deaminase deficiency.

ViroCell will supply viral vectors and gene modified cells, to academic and corporate clients, for translational cell and gene therapies going into clinical trials.

Under the terms of the agreement, Catalent will handle the process development and CGMP manufacturing of AavantiBio’s adeno-associated viral vector-based therapeutic candidate for use in clinical trials in the US and Europe.

Avantor plans to increase its single-use manufacturing footprint by 30% and double its cleanroom space in the US and Europe.

Vertex and CRISPR have amended their collaboration agreement to include the development, manufacture, and commercialization of CTX001, an investigational CRISPR/Cas9-based gene editing therapy for sickle cell disease and transfusion-dependent beta-thalassemia.

Catalent plans to add cryogenic capabilities to its facility in Philadelphia, which will strengthen its ability to cryogenically preserve cell therapies and other biological materials.

The ATMPS and Ori Biotech collaboration aims to improve visibility and reporting of cell and gene therapy manufacturing through a pre-integrated data solution.

The UK's National Horizons Centre has been confirmed as a National Training Centre that will deliver on-site advanced therapies and vaccine manufacturing specific training.

This agreement enhances GSK’s investment in cell and gene therapy manufacturing in the UK for clinical trials.

More work is needed to ensure the rising demand for cell and gene therapy manufacturing capacity and required skilled workforce can be met in Europe.

Under the terms of the acquisition, OXGENE will become a fully owned subsidiary of WuXi Advanced Therapies, WuXi AppTec’s cell and gene therapy contract testing, development, and manufacturing organization business unit.