
Rob Blanchard and Clive Roberts discuss the issues surrounding tablet sticking.

Rob Blanchard and Clive Roberts discuss the issues surrounding tablet sticking.

Pfizer and Mylan have agreed to establish an exclusive long-term collaboration to develop, manufacture, distribute, and market generic drugs in Japan. The products included in the collaboration are expected to be sold under the Pfizer brand with joint labeling.

A Q&A with Babu Padmanabhan, Managing Director and Chief Knowledge Officer of STEER Engineering, on recent industry trends.

A look at elastomer changeout times shows how industry knowledge improves operations and cost.
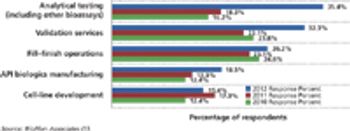
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.
Enhancing bioavailability can be achieved through hot-melt extrusion or spray drying. Patricia Van Arnum interviews Bend Research to find out more about when to use each technique.

Meticulous system configuration can prevent machines from taking over.
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

Nanolipogels for anticancer drug delivery, inhalable and thermo-responsive, fat-encased nanoparticles for targeted drug delivery, and calcium carbonate microspheres are some recent developments.

Dedong Wu, senior scientist at AstraZeneca, explains how nanoparticle engineering and crystal engineering can aid solubility.

MedImmune, the global biologics arm of AstraZeneca, announced that it is restructuring its infectious disease and vaccines R&D and operations. This will result in the closure of MedImmune's sites in Mountain View and Santa Clara, California.

Sandoz announced it has completed the acquisition of Fougera Pharmaceuticals, a maker of generic dermatology products, for $1.5 billion.

On July 9, 2012, Congress passed the Generic Drug User Fee Act in an effort to expedite the process of bringing generic drugs to market. The Act authorizes the collection of user fees from generic-drug manufacturing companies for the first time in the industry's history.

Holistic open learning networks offer a new drug R&D model for improving research outcomes.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.
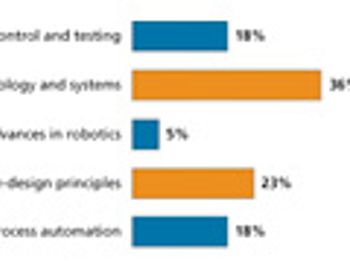
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Advances in targeted drug delivery and customized release profiles are key goals.

Improvements in expression platforms and enhanced tools for selecting clones are among the advances of the past few decades.

Uniform dose formulation is key to meeting safety study requirements.

A look back at key nanoformulation advances and what lies ahead for nanoparticle-based drug-delivery systems.

Gold nanoparticles for targeting tumor sites and elastic capsules using nanosized flakes are some recent approaches used to control and target drug delivery.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2012 edition from EMD Millipore and Meissner Filtration Products.

Novartis, GlaxoSmithKline, and Lonza are among those participating in newly formed consortiums for developing and producing medical countermeasures.