
Fifty percent of the marketing authorization applications for human medicines submitted to the European Medicines Agency (EMA) in 2009 were for generic and hybrid medicines?60% higher than the agency's forecast.

Fifty percent of the marketing authorization applications for human medicines submitted to the European Medicines Agency (EMA) in 2009 were for generic and hybrid medicines?60% higher than the agency's forecast.

Signaling its interest to strengthen its presence in emerging markets, Abbott (Abbott Park, IL) has agreed to acquire Piramal Healthcare Ltd.'s (Mumbai, India) Piramal Healthcare Solutions business (domestic formulations) for $3.72 billion.

Lonza Acquires MODA; Sigma-Aldrich Exec to Retire; And More.

FDA Clears Infant Rotavirus Vaccine

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2010 edition from ATMI and SciLog.

Week Of May 10, 2010: Company And People Notes: Ariad and Merck Restructure Collaboration; Genzyme Changes Leadership; and More

In response to stringent price cuts introduced in Spain, the European Generics Association (EGA) is urging the Spanish government to adopt mechanisms that will help increase the dispensing of generic medicines.

FDA Approves AstraZeneca's and Pozen's Vimovo; Merck Announces Management Changes; And More.

The traditional lines of demarcation between innovator-drug and generic-drug companies are blurring as each sector responds to changing industry fundamentals.

Further consolidation, slowing growth in established markets, and growth in smaller and emerging markets are important factors influencing the direction of the global generic-drug market.

FDA wants industry to talk to them about the science underlying process innovations-really.
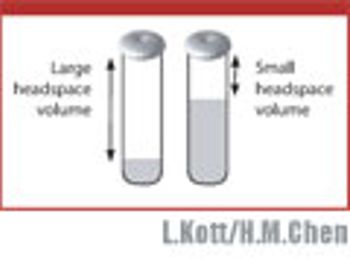
In this case study of amines, the authors discuss several parameters to be considered in developing a headspace GC method.

The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.

The growth of Brazil's generic-drug market is on a fast track, but what are the projections for the sector's future?

A Pharma Business Imperative.

When vessels, seals, and cooling units go haywire, operators must get in the mix.

The author discusses a new fillm-coating system to create customized colors and the research an development tools used in conjunction with this system. This article is part of a special supplement on Excipients and Solid Dosage.

A Technical Forum featuring representatives from Dow Chemical, ISP, and DMV-Fonterra Excipients. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."
The author outlines the key concepts of ISPE's recently revised Baseline Pharmaceutical Guide for New and Renovated Facilities. This article is part of a special supplement on Excipients and Solid Dosage.

The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

New Draft Guidance for Solid Oral Dosage Forms Focuses on Physical Chemical Identifiers. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The authors formulated bupropion hydrochloride tablets with various grades of methacrylic copolymers and analyzed the properties of the resulting dosage forms. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

A new automated factory in Boston (MA, USA) has been developed that uses non-genetically modified green plants to quickly produce large quantities of vaccines and therapeutics.

Charles River Acquires WuXi AppTec; Amgen Appoints President and COO; and More.