Formulation and Drug Delivery
Latest News
Latest Videos

More News
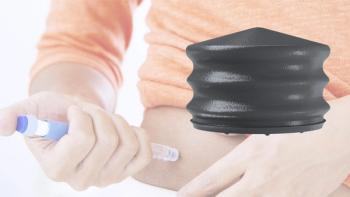
Datwyler has launched new coated plungers in its NeoFlex line that are suitable for large volume biologics.

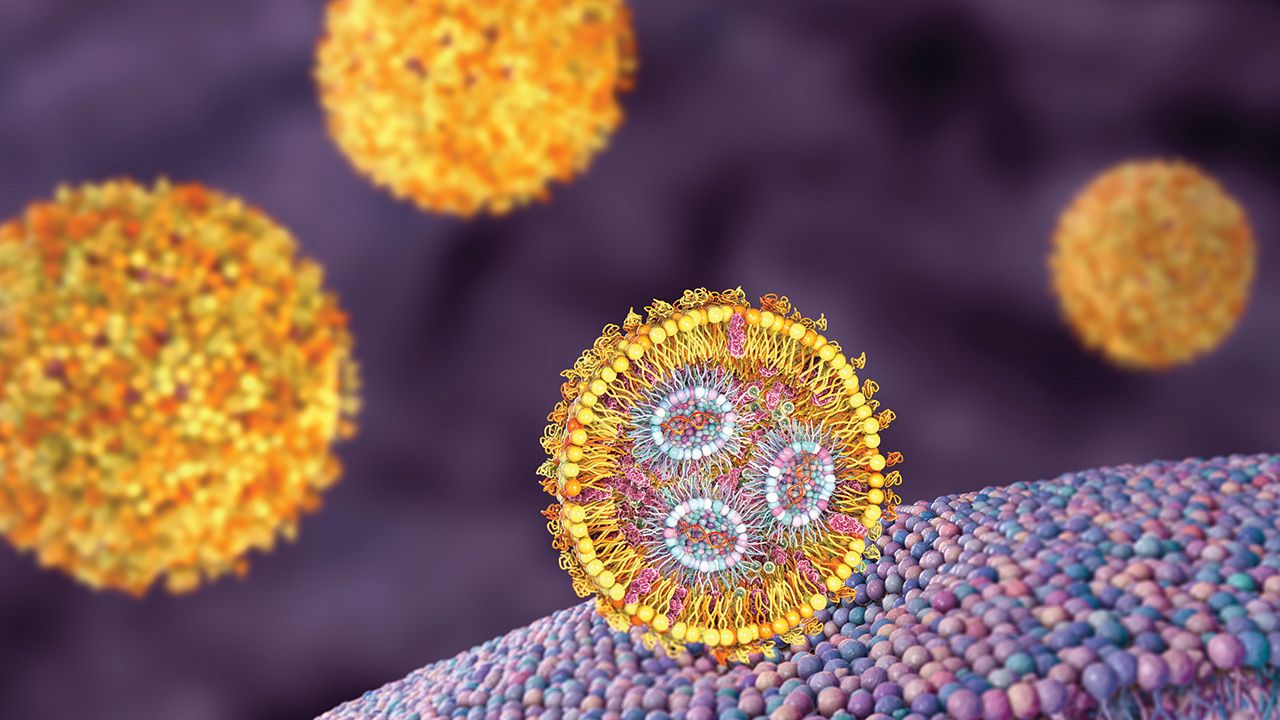
The companies will collaborate to create and test circVec DNA–LNP formulations for use in potential therapeutic applications.

The partnership will use ViaNautis’ proprietary polyNaut technology platform to develop genetic medicines that can precisely target tissue types.

WHO and its partners are providing support to countries dealing with mpox outbreaks.

The report highlights international regulators’ plans for the development and availability of vaccines to prevent and drugs to treat mpox.

Pfizer’s bivalent RSV prefusion F vaccine has been approved to prevent lower respiratory tract disease resulting from the respiratory syncytial virus in adults 18 to 59 years of age who are at increased risk.

For the pharmaceutical industry, drug formulation is a cornerstone, crucial for converting bioactive molecules into effective, stable, and patient-friendly medications.

Evonik won the CPHI Excellence in Pharma Award in the “Sustainability” category in recognition of its plant-based squalene, PhytoSquene, used in parenteral drug delivery applications.

Pharmaceutical Technology sat down with Niloufar Salehi, advisor at Eli Lilly & Company, to talk about the session she is moderating at AAPS PharmSci 360 2024, Symposium: An Accelerated Development of Poorly Soluble Drugs Using Predictive Tool.

Sustainability of small-molecule API manufacturing ensures continued success.

The best strategy is to use a combination of complementary methods.

Solving the challenge of better-stabilized temperature-sensitive biomolecules hinges on innovative formulation strategies.

A new Center of Excellence will be established at PCI Pharma Services’ location in Rockford, Ill., and two sites in Ireland will also benefit from the investment.

FDA’s Darby Kozak provided commentary on the anniversary of the Drug Price Competition and Patent Term Restoration Act of 1984.

The agency is recommending the extension of the smallpox and mpox vaccine, Imvanex, to adolescents aged 12 to 17.

Excipient and delivery device selection play crucial roles in the formulation of inhaled drugs.

New and existing technologies, as well as a patient-centric focus, are pushing drug formulation into exciting directions.

Vectura’s expertise in the field includes formulation and device development for dry powder inhalers and pressurized metered dose inhalers, among other products and services.

The company said this new commitment to its facility in Germany goes hand in hand with its recently announced ReciPredict, an initiative intended to streamline the product development cycle.

Insights into molecular behaviors and predictive capabilities are bringing numerous benefits.

A coordinated and international response is needed to help control the latest mpox outbreak in Africa.

For a preview of what's to come at the AAPS PharmSci 360 show in October, Pharmaceutical Technology® spoke with Vivek Gupta, PhD, associate dean for Graduate Education and Research, associate professor, Pharmaceutical Sciences, St. John’s University, Queens, New York, about the formulation of inhalation dosage forms.

Employing novel technologies and more patient-centric approaches can help to reduce the potential of formulation failure.

Biopharmaceutical production faces the challenge of ensuring the quality of raw materials due to a lack of specific guidelines. By implementing effective risk assessment strategies and working with reliable, selected solution providers, biopharmaceutical manufacturers can minimize these challenges and improve product quality.

A holistic approach to automation can provide benefits at all stages of development and manufacturing.