
With the $252-million acquisition of contract development, manufacturing, and testing organization Avista Pharma Solutions, Cambrex will enter the market for early stage small-molecule development and testing services.

With the $252-million acquisition of contract development, manufacturing, and testing organization Avista Pharma Solutions, Cambrex will enter the market for early stage small-molecule development and testing services.

In preparation for the upcoming US Drug Supply Chain Security Act deadline, Metrics Contract Services has expanded serialization capabilities at its oral solid-dose commercial manufacturing site in Greenville, NC.

Experts believe that the contract development and manufacturing organization market will reach $17.38 billion by 2022, with disruptive business models using Industrial Internet of Things (IIot) and single-use technologies proving more profitable and efficient in the long term.

Pharmatech Associates International Contracting and Services is a new company headquartered in Doha, Qatar, that will provide product development, facility design, and construction for high quality pharmaceutical and biotech products at low cost.

Idifarma, a contract development and manufacturing organisation (CDMO), based in Spain, has announced the commencement of the seventh project with Palobiofarma-a Spanish biotechnology company.

The contract development and manufacturing organization released its first serialized products to Europe from its facilities in Lisbon, Portugal and Stockholm, Sweden.

The new facility will include comprehensive mammalian process development and manufacturing capabilities.

Industry experts discuss the formulation and development issues that should be considered when addressing scale up from small-scale batches to commercial production.

The sterile-manufacturing contract development manufacturing organization is approved by FDA for viral vector manufacturing fill/finish processing at its biologics facility in Scotland, UK.

The contract development and manufacturing organization expanded its analytical chemistry suite and added a new office in Boston, MA.

PCI Pharma Services and CSP Technologies will partner on protective packaging solutions for clinical trials and stability testing.

Increasingly complex trial protocols have added to IMP manufacturing challenges.
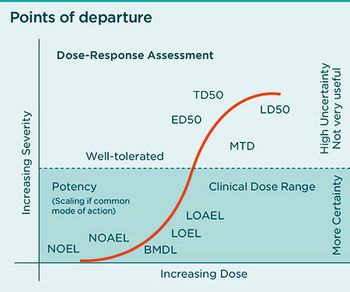
Determining how much containment is needed for API handling requires evaluation of multiple factors.

This article provides a sampling of the latest investments, expansions, and acquisitions by small-molecule contract service providers.

Heightened uncertainty means CDMO executives need to play out planning scenarios.

The glass and chemical provider will expand its synthetic pharmaceutical intermediate and API production capacity at its plant in Chiba, Japan.

Catalent’s acquisition of Juniper Pharmaceuticals further expands its early development capabilities.

The company expanded its extended workbench laboratory services program to support the ongoing manufacturing and development of Flexion Therapeutics’s Zilretta (triamcinolone acetonide extended-release injectable suspension).

The extension provides four additional process development laboratories at its Nottingham site to improve output and efficiency in drug development and clinical trial manufacturing operations.

WuXi STA supported the development of hepatitis drug through marketing authorization holder pilot program.

Vetter anounced the Open Innovation Challenge to examine the applicability of digital trends to injection systems.

Under the contract, AMRI will focus on the development and manufacture of APIs and drug product for Phase I clinical studies.
From separation systems to reactor technology, new tools are increasing the feasibility of continuous API production.

Madison Dearborn Partners will acquire a majority ownership position in CDMO Alcami.

Catalent’s GPEx technology was used to develop antibody for anti-methamphetamine clinical study.