
Catalent will expand primary packaging capabilities and commission an automated, high-speed bottling line for clinical packaging of capsules and tablets.

Catalent will expand primary packaging capabilities and commission an automated, high-speed bottling line for clinical packaging of capsules and tablets.

The company will complete an expansion of its secondary packaging capabilities at its Ravensburg, Germany site by 2020.

The contract development and manufacturing organization released its first serialized products to Europe from its facilities in Lisbon, Portugal and Stockholm, Sweden.

PCI Pharma Services and CSP Technologies will partner on protective packaging solutions for clinical trials and stability testing.

ICS distribution center will serve manufacturers of specialty medications, biosimilars, and cell and gene therapies.

Rommelag will showcase its single-use, flexible packaging system at Achema 2018.

Rommelag will showcase its blow-fill-seal technology that collectively offers container production, aseptic filling, and container closure.

Shipping biopharmaceuticals in single-use containers requires a thorough understanding of the distribution cycle and potential transportation risks.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

Grand River Aseptic Manufacturing announces equipment purchase to add vial filling capacity.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

Automation systems, vehicles, and robots improve efficiency of transporting materials and finished goods in pharmaceutical warehouses.

The company has officially opened a local brand office in South Korean to support its existing business in the region.

The contract development and manufacturing organization (CDMO) Aesica Pharmaceuticals has had a serialization program in place for the past five years, and recently installed capabilities for serialization at all its packaging facilities.

The suite installation increases PCI’s Hay-on-Wye site’s serialization capability to support clients in advance of meeting the implementation dates of the European Falsified Medicines Directive.

Sharp acquired the pharmaceutical packaging facility in Bethlehem, PA.

Cold-chain requirements and the tight logistical windows needed for cell and gene therapies demand a focus on early communication and risk mitigation.
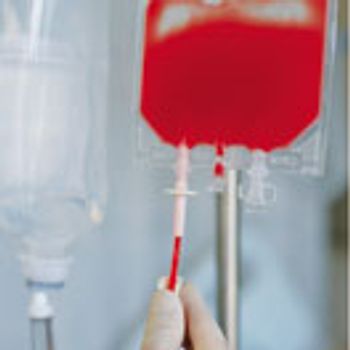
Andrea Zobel, senior director of product management, clinical trial supply, and logistics at PAREXEL International, examines key issues and questions that sponsors and contract partners must address.

Many pharmaceutical manufacturers were late in involving contract partners in serialization efforts. Are you ready, and are you working with the right partner?
As the date for implementation of the Drug Supply Chain Security Act approaches, bio/pharma companies and contractors should focus on key areas.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

The company has manufactured double-digit number of batches on the new filling line, for use in early-stage clinical trials.

The company expanded its commercial packaging facility in response to a growing demand for pediatric drugs.
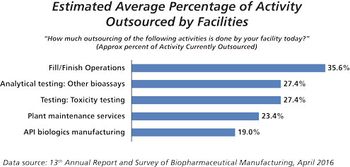
This key bioprocessing segment is expecting continued growth

Now past the “wait-and-see” standoff, most pharmaceutical companies and their contract partners are considering long-term requirements, including distributors’ needs.