
FDA cites Yunnan Hande Bio-Tech for cGMP violations related to data collection and security.

FDA cites Yunnan Hande Bio-Tech for cGMP violations related to data collection and security.

An integrated pilot plant tests heteronucleation and continuous crystallization.

Recipharm makes a strategic investment in Synthonics and partners in development of novel compounds.

New legislation and changes in policy at FDA are leading to better control of the API supply chain.

A consensus-based standard issued by NSF International incorporates regulatory and industry requirements into a single standard for the manufacturing and distribution of pharmaceutical excipients.
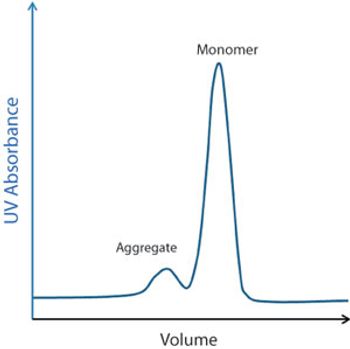
Several chromatographic resins are available for downstream purification.

Taste may be subjective, but it is crucial to patient compliance, particularly for pediatric treatments.

Analytical technologies must accurately identify and measure the critical material attributes of APIs and excipients.

Ionic liquid technologies offer a new way to improve bioavailability.

New cellulosic polymers have been shown to improve solubility in these key amorphous solid dispersion processes.

Protecting workers, patients, and the environment requires advanced technologies.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

Several chromatographic resins are available for downstream purification.

It is vital that companies involved in the manufacturing and handling of cytotoxic drugs ensure that staff are given the highest possible levels of protection.
Resolution technologies remain crucial for commercial-scale chiral API production.

CMO executives share their opinions on where outsourcing is going and what is driving market change.
After launching a new mammalian cell platform, FUJIFILM Diosynth Biotechnologies U.S.A., has acquired fast-track vaccine manufacturing knowhow and a major presence in Texas’ emerging biocorridor with Kalon, its first acquisition.

New drugs submitted for approval in Europe have 18 months to comply with new elemental impurities guidelines.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

USP establishes Jan. 1, 2018 as the implementation date for its elemental impurities guidelines for existing drugs.

Changing dynamics of the pharmaceutical industry are driving demand.

Analytical and procedural deficiencies result in FDA warning letter for Novacyl Wuxi Pharmaceutical.

Protein Sciences will evaluate sourcing Flublok from its Japanese licensee, UMN Pharma, which already runs a large-scale facility for the vaccine.

FDA approves treatments for new diseases and drugs that operate by new mechanisms.