
The new drug, Onpattro (patisiran), by Alnylam Pharmaceuticals, is in a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

The new drug, Onpattro (patisiran), by Alnylam Pharmaceuticals, is in a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

The companies will collaborate on the discovery, development, and commercialization of cell therapies for cancer.

Low-temperature chemistry enables performance of more challenging and selective chemistry.
Development costs and time to market continue to put pressure on the biopharma industry, driving the need for innovation in methods and technologies.
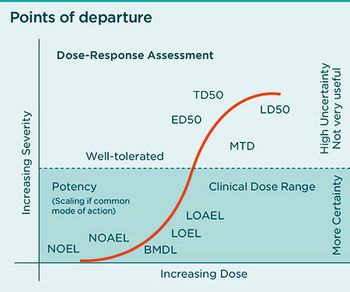
Determining how much containment is needed for API handling requires evaluation of multiple factors.

CMOs have been active over the past year in expanding their biologics production and capabilities.

The acquisition will place Cambrex into the finished dosage form CDMO market.

The company has run into a snag in producing Kymriah for the diffuse large B-cell lymphoma patient population, the second indication for which the therapy was recently approved by FDA.

CELLforCURE will produce cancer CAR-T treatments for Novartis at a manufacturing facility in Les Ulis (Essonne), France.

The media, by Tosoh Bioscience, is composed of calcium and phosphate buffers and offers mixed-mode properties for biomolecule purification.

Both parties have agreed to recognize each other’s inspection of manufacturing sites for a broader range of medicines, including sterile products, APIs, biologics, and vaccines.

The agency is releasing six new draft guidances to provide a regulatory framework for handling gene therapies.

Report predicts PAT, NIRS, continuous bioprocessing, and a ‘technological arms race’ could improve biopharma manufacturing efficiencies.

After 30 years of biologic-drug advances, the industry and patients still have a lot to learn.
Biopharma seeks alternatives that meet the needs for next-gen biologic drug production.

With the right excipients, formulators can control when, where, and how an API is released.

Minakem’s facility in Belgium enhances capacity to scale production of highly potent ingredients for small to full GMP batches.

The company has completed the first phase of expansion at its headquarters in Freiburg, Germany, in anticipation of increasing demand as cell and gene therapies approach commercialization.

The three-year collaboration will focus on developing vaccines for up to five infectious disease pathogens.

The contract development and manufacturing company has received an additional approval from Health Canada to manufacture monoclonal antibody drug substance at its first plant in Icheon, South Korea.

Cambrex expands its generic API research and development capabilities at its Milan, Italy site.

The company will provide the first FlexFactory manufacturing platform for cell therapy manufacturing.

Under the contract, AMRI will focus on the development and manufacture of APIs and drug product for Phase I clinical studies.

The agency has recommended approval of three biosimilar adalimumab products from Novartis, referencing AbbVie’s Humira, and a biosimilar trastuzumab from Pfizer, referencing Roche’s Herceptin.

EMA has recommended marketing authorization for Aimovig (erenumab), a new treatment for migraine.