
The EMA has issued a statement addressing the withdrawal of the marketing authorization for Thelin, which was voluntarily withdrawn in 2010 because of a risk of serious liver injury.

The EMA has issued a statement addressing the withdrawal of the marketing authorization for Thelin, which was voluntarily withdrawn in 2010 because of a risk of serious liver injury.

In France, public confidence in health authorities and pharmaceutical industry has been shaken by a safety scandal over the drug Mediator (benfluorex hydrochloride).

FDA announced on Mar. 2, 2011, that it is taking action against companies that manufacture, distribute, or market certain unapproved prescription oral cough, cold, and allergy products, according to an agency release.

Fujifilm and Merck & Co. have formed a definitive agreement by which Fujifilm will acquire the Merck BioManufacturing Network, a contract biopharmaceutical manufacturing and development business of Merck.

A number of European public health and transparency campaigners believe that conflict of interest rules may have been breached with the EMA's decision to allow its former Executive Director, Thomas Lönngren, to take up an advisory role within the private pharmaceutical sector.

The European Organization for Rare Diseases (EURORDIS), an alliance of patient organizations and individuals, celebrated its fourth Rare Disease Day on February 28.

FDA and Georgetown University Form Innovation Partnership

Pfizer Completes King Acquisition; ViiV Healthcare Names Chairman; and More.

Ongoing Free Trade Agreement (FTA) negotiations between the EU and India have hit a hurdle as some stakeholders urge the Indian government to fight against certain provisions in the FTA amid fears that access to generic drugs may be affected.

FDA recently sent sanofi-aventis two Warning Letters for its facilities in Frankfurt am Main, Germany, and Bridgewater, NJ.

Brazil develops its first national plasma fractionation plant.

A single, global tooling standard would offer many benefits, but one has been slow to emerge.

Social media tools have taken over many aspects of our lives, now including regulatory info.
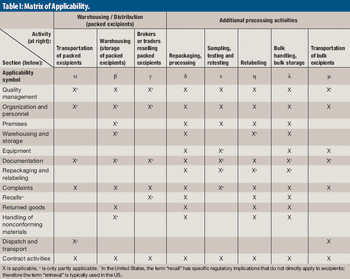
A new audit guide aims to improve supply-chain security and supplier qualification practice.

Just when things seem to be looking up, the unexpected problem occurs.

FDA's efforts to improve access to treatments for rare diseases.

INTERPHEX 2011 aims to address the industry's unique characteristics.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

Bristol-Myers Squibb opens a new pediatric HIV/AIDS clinic in Tanzania.

This article is Part I of a three-part series on biopharmaceutical issues in public health, government, and developing-world markets. Part 1 focuses on drug-development. Part II, which examines manufacturing, appeared in the April 2011 of Sourcing and Management. Part III, which analyzes distribution and administration,appeared in the May 2011 issue.

After reviewing further data on the suspected link between cases of narcolepsy and GlaxoSmithKline's pandemic influenza vaccine, Pandemrix, the EMA's Committee for Medicinal Products for Human Use (CHMP) is still unable to establish a causal relationship.

An initiative based in the Netherlands aims to bring together universities, pharma companies, international research institutions and product development partnerships to help develop new diagnostic tests and medicines for neglected tropical diseases.

Pharma's manufacturing and supply chain needs a "radical overhaul" because it is underused, inefficient and ill-equipped to cope with new types of products that will be coming to market in the near future, according to a report from PricewaterhouseCoopers (PwC).

The Society of Chemical Manufacturers and Affiliates (SOCMA), in a statement submitted to the US House Subcommittee on Cybersecurity, Infrastructure Protection and Security Technologies, called for Congressional action to pass a three-to-five year authorization of current Chemical Facility Anti-Terrorism Standards (CFATS), which are scheduled to expire Mar. 4, 2011.

Gilead to Acquire Calistoga; Bayer Healthcare Appoints Former Pfizer Exec; and More.

HHS Releases a New National Vaccine Plan

Last Wednesday, sanofi aventis agreed to acquire Genzyme for $74 per share in cash, or approximately $20.1 billion.

President Barack Obama released his budget proposal for fiscal year (FY) 2012, which shows an increase in funding for the US Food and Drug Administration.

EMA Outlines Activities for 2011, and more.