
Merck & Co. Resolves Vioxx Lawsuit; Takeda Agrees to Acquire Envoy Therapeutics; and More.

Merck & Co. Resolves Vioxx Lawsuit; Takeda Agrees to Acquire Envoy Therapeutics; and More.

Pharmaceutical companies and public health officials gather at the IFPMA Assembly to urge for greater innovation and private and public partnerships to address global health concerns.

A roundup of developments in corporate social responsibility (CSR) and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

The European Commission has issued final approval for Europe's first gene therapy-a treatment for a rare genetic disorder that currently has no other treatment options.

Watson Pharmaceuticals has completed its EUR 4.25 billion ($5.4 billion) acquisition of the Actavis Group. The combination creates the world's third largest generic-drug pharmaceutical company, with anticipated pro forma combined 2012 revenues in excess of $8 billion, according to Watson.

FDA pushes back goals due to Hurricane Sandy and EMA announces changes to variation regulations.

Boehringer Ingelheim has agreed to pay $95 million to settle allegations that it improperly promoted four of its drugs.

USP revises labeling requirements for Heparin.

A Q&A with Yves de Montcheuil, vice-president of marketing at Talend, a provider of open-source integration software.
Consider critical process parameters and strategies to optimize the manufacturing process.

Even when all is well at the facility, one must expect the worst while braving the elements.

Key talks from the recent PDA/FDA regulatory conference highlight room for improvement.

The government of Turkey is drawing up a program in coordination with the pharmaceutical industry to create ways to make the country a regional production center for pharmaceuticals serving Europe, Central Asia, and the Middle East.
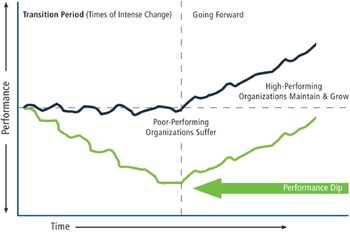
A disciplined approach to changing behavior can achieve change agility.

Overt and covert packaging technologies evolve to authenticate drugs and fight counterfeits.

Global tactics that incorporate online technologies and social media are reshaping disease response.

Novartis to Construct New Biotechnology Facility in Singapore; Patheon to Acquire Banner Pharmacaps; and More.

Patheon will acquire softgel-specialist company Banner Pharmacaps, based in North Carolina in the US, for $255 million

Novartis is investing more than half a billion US dollars in the construction of a new state-of-the-art biotechnology production site in Singapore for the manufacturing of drugs based on cell culture technology.

AET BioTech and BioXpress Therapeutics have entered into an agreement to codevelop a biosimilar version of Abbott's tumor necrosis factor inhibitor monoclonal antibody adalimumab.

Pharmaceutical industry leaders Novartis and Merck-among others-released second-quarter 2012 results showing global sales down in third-quarter 2012, with growth in key pharmaceutical products helping to offset losses due to patent expirations.

New England Compounding Center gets 483 after linked fungal meningitis outbreak.

Pfizer Announces Intention to Acquire NextWave Pharmaceuticals; Seattle Genetics Expands ADC Collaboration with Abbott; and More.

On Oct. 22, 2012, a consortium in France announced the establishment of Europe's first ever industrial manufacturing facility dedicated to the large-scale production of novel, advanced cell-based therapy medicinal products.

FDA announces Coalition for Accelerating Standards and Therapies and Commissioner Hamburg comments on meningitis outbreak.

Ben Venue Laboratories has resumed production on a limited number of manufacturing lines in the company's Bedford, Ohio, facilities.

Watson Pharmaceuticals has received clearance from the Federal Trade Commission for its acquisition of generic drug manufacturer, Actavis.

Dr Reddy's is planning to acquire the specialty injectable company OctoPlus for approximately EUR 27.4 million ($35.7 million) in cash to strengthen its technological capabilities in drug delivery.

Catalent Licenses NJIT Taste-Masking Technology; MicroConstants Completes FDA Inspection; and More.

EMA releases guideline on medicinal products for the treatment of schizophrenia.